CASE REPORT
JOP. J Pancreas (Online) 2011 May 6; 12(3):274-278.
Recurrent Pancreatic Pseudocyst Diagnosed 9 Years After Initial Surgical Drainage
Carlos M Nuño-Guzmán, José Arróniz-Jáuregui, José I Gómez-Ontiveros, Haydée Hernández- Estrada, Haydee I Estrada-Castañeda, Juan R Araiza-Navarro, Nereida Esparza-Arias
Department of General Surgery, Antiguo Hospital Civil de Guadalajara “Fray Antonio Alcalde”. Guadalajara, Jalisco, México
ABSTRACT
Context A pancreatic pseudocyst is defined as a collection of pancreatic juice enclosed by a wall of fibrous or granulation tissue which is not lined by epithelium. Acute pseudocysts occur in acute pancreatitis but can be found after an acute exacerbation of chronic pancreatitis. Chronic pancreatic pseudocysts are typically found in chronic pancreatitis but may develop after an occurrence of acute pancreatitis as well. Most acute fluid collections and pseudocysts will show spontaneous resolution while the remaining may persist with or without symptoms, or progress to produce complications. Treatment is indicated for persistent, symptomatic pseudocysts and, in the case of complications. There is no clear consensus regarding the optimal clinical or radiologic follow-up after treatment. Detection of late recurrence is not common, and the possibility of a cystic neoplasm must be ruled out. Case report We report the case of a 67-year-old female patient who was referred to our institution as the result of a pancreatic pseudocyst. The patient had presented a pancreatic pseudocyst 9 years earlier which had been surgically treated by a cystogastrostomy. No additional acute pancreatic episodes occurred. The diagnostic and treatment approach of this unusual late recurrent pancreatic pseudocyst is herein described. Conclusion The unusual late presentation of a recurrent pancreatic pseudocyst requires clinical, laboratory and radiological evaluation. In the present case, the clinical background, amylase fluid levels and tomographic findings were highly suggestive of a pancreatic pseudocyst.
INTRODUCTION
A pancreatic pseudocyst is defined as a collection of pancreatic juice enclosed by a wall of fibrous or granulation tissue which is not lined by epithelium [1]. Pseudocysts represent about two-thirds of pancreatic cystic lesions, found in 10-20% of acute pancreatitis cases and 20-40% of chronic pancreatitis patients. Most acute fluid collections and pseudocysts will show spontaneous resolution while some may persist with or without symptoms, or progress to produce complications. Treatment is indicated for persistent, symptomatic pseudocysts, and in the case of complications [2, 3]. There is no clear consensus regarding the optimal clinical or radiologic follow-up after treatment. Detection of late recurrence is not common, and the possibility of a cystic neoplasm must be ruled out.
CASE REPORT
A 67-year-old woman was referred to our department with the presence of an abdominal mass in the upper abdomen, detected during a routine gynecologic visit. She had had an episode of acute pancreatitis 9 years earlier, with the subsequent development of a pancreatic pseudocyst, which was surgically managed by a cystogastrostomy 2 months later in another institution; a cholecystectomy was performed as well. She denied any episode of abdominal pain thereafter and did not recall post-surgical follow-up beyond one year. There was no history, or clinical nor laboratory data of diabetes or exocrine pancreatic insufficiency. Physical examination revealed a midline surgical scar, minimal tenderness upon palpation of a non-mobile mass in the upper abdomen. Laboratory serum results showed no abnormalities. The abdominal computed tomography (CT) scan revealed a 135x58 mm well-circumscribed unilocular cystic lesion in the region of the pancreas (Figure 1).
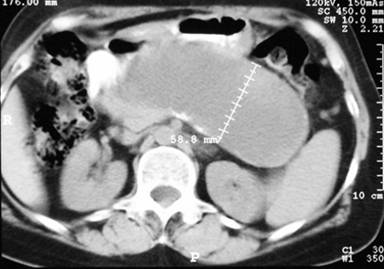
|
Figure 1. CT scan showing a well-circumscribed unilocular pancreatic pseudocyst in close apposition to the posterior gastric wall. |
The diagnosis of pancreatic pseudocyst was highly probable but, considering the uncommon chronic presentation, and, in order to measure amylase, tumor marker CA 19-9 and carcinoembryonic antigen levels (CEA) in the cystic fluid, an image-guided puncture was planned. To demonstrate possible communication between the pseudocyst and the main pancreatic duct, an endoscopic retrograde cholangiopancreatography (ERCP) was planned as well but, since the patient was reluctant to undergo both procedures, surgical drainage was carried out instead. Since the pseudocyst appeared to be adherent to the posterior gastric wall on CT scan, a cystogastrostomy was scheduled. At laparotomy, by means of an anterior gastrotomy and a 5 cm incision using electrocautery at the posterior gastric wall, where the bulging pseudocyst was visualized, abundant non-viscous clear brownish fluid was drained immediately. A fluid sample and pseudocyst wall biopsy were sent for determination of amylase level and histopathological exam. The amylase fluid level was 8,524 IU/L. The cystogastrostomy was completed using a running 2-0 polypropylene suture. The anterior gastrotomy was sutured as was the abdominal wall. Both frozen section and definite histopathological examination demonstrated fibrous tissue, with no epithelial lining, epithelial tissue or malignant transformation. With an uneventful recovery, the patient was discharged 10 days after surgery. At follow-up 12 months later, an abdominal wall protrusion was noted upon exertion. An abdominal wall defect was palpated at the surgical scar. A control CT scan demonstrated a midline abdominal wall defect, but no evidence of a pancreatic pseudocyst (Figures 2 and 3). Incisional hernia surgery was performed. Six months after this latter surgery, the patient remains in good condition.
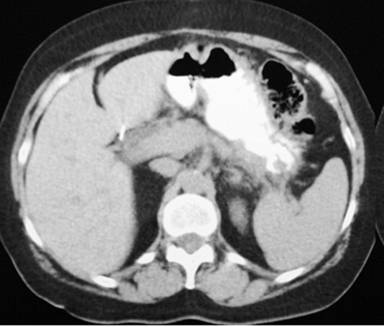
|
Figure 2. Pancreatic CT scan with absence of a pseudocyst. |
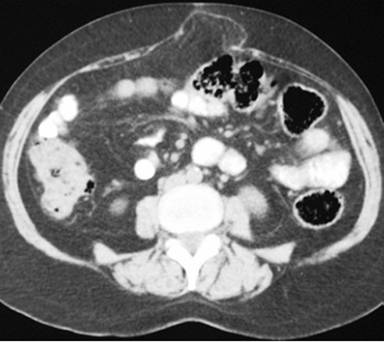
|
Figure 3. Midline abdominal wall defect with partial protrusion of the omentum. |
DISCUSSION
The natural history of pancreatic pseudocysts following acute pancreatitis is well known; while about 40% of acute fluid collections show spontaneous resolution within 6 weeks, a thick wall of granulation tissue takes 4 to 6 weeks to develop around the remaining 60%. The pancreatic pseudocyst may then regress spontaneously, persist with or without symptoms, or progress to produce complications [3]. In contrast, pancreatic pseudocysts do not follow the same rate of spontaneous resolution in chronic pancreatitis [4]. Regression rates for asymptomatic chronic pancreatic pseudocysts vary between 9 and 31% [2, 5]. Acute pancreatitis, small pseudocyst size, intrapancreatic location, pseudocyst of the head of the pancreas, persistence for less than 6 weeks and a thin wall have been associated with spontaneous resolution whereas chronic pancreatitis, persistence for more than 6 weeks, a wall thickness greater than 1 cm, lack of communication with the Wirsung duct, proximal ductal stenosis and an increase in size on follow-up are associated with failure to resolve [2, 6, 7]. Acute pseudocysts occur in acute pancreatitis but can be found after an acute exacerbation of chronic pancreatitis. Chronic pancreatic pseudocysts are typically found in chronic pancreatitis but may develop after an occurrence of acute pancreatitis as well [8, 9].
Although not specific, the most common clinical manifestations are abdominal pain (76-94%), early satiety, nausea and vomiting (50%), weight loss (20-51%), and obstructive jaundice (20%). Upper abdominal tenderness and epigastric fullness (60%), or an abdominal mass may be present [2, 10, 11, 12, 13]. Although larger pancreatic pseudocysts may be asymptomatic, there is a 10% risk of developing complications such as bile duct or duodenal compression/stenosis, rupture, portal hypertension secondary to compression of one or more of the hepatic portal, superior mesenteric and splenic veins, pseudoaneurysm, hemorrhage, pancreatic ascites and infection [2, 10].
When an acute fluid collection results after an episode of acute pancreatitis, and persists on serial imaging over a period of weeks, the diagnosis of an acute pseudocyst is almost certain. But, even in patients who have suffered from acute pancreatitis or a pancreatic pseudocyst, detection of late recurrence is not common, and the possibility of a cystic neoplasm must be ruled out.
Laboratory serum test utility may be limited in the diagnostic approach. White blood cell count, and amylase and lipase levels may be elevated. Serum bilirubin and liver function tests may be above normal values as well [10, 13].
Diagnostic evaluation may begin with a transabdominal ultrasonography. Sensitivity rates in pancreatic pseudocyst detection range between 75% and 90%, usually showing an echoic structure and distal acoustic enhancement contained within a smooth wall [10]. Abdominal CT is the method of choice, showing a thick-walled, round or oval, fluid-filled unilocular mass adjacent to the pancreas which, in a patient with a history of acute or chronic pancreatitis, is virtually pathognomonic. CT has a sensitivity of 82 to 100% and specificity of 98%. An important weakness of the CT scan is its relative inability to differentiate a pseudocyst from a cystic neoplasm [7, 10]. Endoscopic ultrasound (EUS) is the test of choice for distinguishing a pancreatic pseudocyst from a cystic neoplasm of the pancreas. A cyst wall thickness of more than 3 mm, macroseptation, the presence of a mass or nodule, and cystic dilatation of the main pancreatic duct are highly suggestive of a cystic neoplasm. EUS may also be used together with fine needle aspiration (FNA) of the cyst for laboratory evaluation [10].
Analysis of the cystic fluid is particularly useful in differentiating pancreatic pseudocysts and cystic tumors. High amylase levels are typical in pancreatic pseudocysts while low levels are seen in serous cystadenomas; values above 5,000 U/mL show 94% sensitivity and 74% specificity [14]. Carcinoembryonic antigen (CEA) in the cystic fluid has been shown to have low levels in pancreatic pseudocysts and serous cystadenomas while it has elevated levels in mucinous cystadenomas [10, 14, 15]. Although cytological analysis by FNA may be useful for mucinous cysts but of limited value for serous cystadenomas, the potential risk of tumor seeding must be considered. Even intraoperative biopsy of the cystic wall for frozen section histopathology may be unreliable (incorrect in 20% or more of cases) [2]. The absence of epithelial lining or epithelial tissue in pseudocysts excludes a diagnosis of cystic neoplasm [16].
Treatment is indicated in symptomatic cases, manifested as a persistent or recurrent feeling of fullness, early satiety, abdominal distention, nausea or vomiting, and pain or upper gastrointestinal bleeding. Intervention is mandatory in case of complications: compression of major abdominal vessels; compression of the stomach, duodenum or main bile duct; pancreatic ascites or a pancreatopleural fistula; infection and hemorrhage [2, 7, 10]. Treatment is indicated in asymptomatic pseudocysts with the following relative risk factors: pseudocysts greater than 4 cm, no evidence of regression after 6 weeks, a thick capsule of more than 5 mm, chronic pancreatitis, main pancreatic duct stones or strictures and suspicion of a neoplastic cyst [2, 7].
Percutaneous drainage is the least invasive modality, but requires an external drainage catheter which can be placed under US or CT guidance. Recent studies have reported disappointing results for drainage in 40 to 60% of cases. Those patients treated by percutaneous drainage tend to have higher morbidity and mortality rates when compared to surgery [2, 17].
Endoscopic drainage of pseudocysts is a widely used therapeutic modality, either by means of a transpapillary approach using ERCP or by means of a transmural route through the stomach or duodenal wall. A transpapillary approach is possible only if the pseudocyst communicates with the pancreatic duct, which occurs in 36 to 69% of patients. The clinical success of transpapillary drainage ranges from 80 to 100%, with morbidity ranging from 10 to 20%, and recurrence rates ranging from 10 to 20%. No procedure-related mortality has been reported with this approach [2, 10, 17]. With the transmural approach, access to the cyst cavity is achieved through an incision made in the gastric or duodenal wall. The pseudocyst must be in close apposition to the stomach or duodenum, and an area of bulging by luminal compression will mark the location of the pseudocyst. However, this bulging is present in only 42 to 45% of cases. Endoscopic ultrasound-guided drainage has the advantage of not relying on bulging to locate the site of the pseudocyst and excludes the presence of interposed blood vessels. The technical success rates in non-EUS-guided transmural drainage range from 70 to 100%, with morbidity rates ranging from 0 to 33% and recurrence rates ranging from 0 to 30%. When performed using EUS guidance, technical success rates range from 95 to 100%. The endoscopic success rates range from 80 to 100%, and do not vary whether or not EUS is used [2, 10, 17]. The collective data regarding endoscopic drainage show mortality rates of 0 to 1%, long-term follow-up success rates of 62 to 75% of cases and recurrence rates with long-term follow-up of 0 to 23% [2, 9].
Surgical drainage is accomplished by creating communication between the pseudocyst and the stomach, duodenum or jejunum. There are several surgical options. The primary considerations are the anatomic location, the size of the pseudocyst and the degree of chronic pancreatic disease. Wide internal drainage is the most effective therapy for most patients. Surgical drainage is the reference standard, with a success rate of 70 to 100%, morbidity of 6 to 37%, mortality of 1 to 16% and recurrence rates of 2 to 30%. [2, 9, 12]. Surgical drainage options include pseudocystogastrostomy, pseudocystoduodenostomy and pseudocystojejunostomy. A pseudocystogastrostomy is recommended for pseudocysts directly adherent to the posterior gastric wall. Small pseudocysts (less than 4 cm) in the head and uncinate process of the pancreas impinging on the duodenal wall are best managed by a pseudocystoduodenostomy while a pseudocystojejunostomy is a good surgical option for all other pseudocysts [2, 18]. Minimally invasive pseudocystogastrostomy and pseudocystojejunostomy provides adequate internal drainage. Case series suggest that the laparoscopic approach is safe, although there is still a lack of definitive evidence [18, 19]. No prospective, controlled study has compared surgical, percutaneous and endoscopic drainage results.
After a definitive treatment of pseudocysts, surveillance imaging with US, CT or MRI is often used. If the symptoms do not improve after treatment, follow-up imaging is warranted. There is no clearly defined value for a longitudinal follow-up after treatment in an asymptomatic patient [19].
Grewal et al. reported the case of a 45-year-old man with epigastric pain and a 10-year history of numerous episodes of acute pancreatitis secondary to alcohol abuse [20]. Nine years previously, a pancreatic pseudocyst had been drained by a surgical cystogastrostomy. After conservative treatment, symptom recurrence and pyloric obstruction, a 5.5x5.3x6.0 cm pseudocyst was drained utilizing endoscopic “needle-knife” cystogastrostomy, upon reluctance on the patient to accept additional surgery.
Struve reported the case of an 82-year-old woman having spontaneous resolution of a 7 cm pancreatic pseudocyst 10 years after detection which had been followed by ultrasonography since the patient refused percutaneous or surgical management, due to the absence of symptoms [21]. During the 10-year period, no recurrence of pancreatitis was noted.
In the case herein reported, after the acute pancreatitis episode and the initial pseudocyst surgical drainage, there were no further episodes of abdominal pain reported by the patient. The lack of symptoms and of clinical or radiological post-surgical follow-up makes it impossible to determine the time of recurrence. The CT findings were also suggestive of a pancreatic pseudocyst which made surgical treatment the most appropriate option for drainage and biopsy of the cyst wall.
In our hospital, a public institution, EUS is not available, and access to it in our city is still very limited. Once available, it will constitute an ideal diagnostic modality with a known potential for guiding endoscopic transmural drainage. The high intraoperative amylase cyst fluid level was also highly suggestive of a pseudocyst.
In summary, we reported an unusual late presentation of a patient having a recurrent pancreatic pseudocyst, with no symptoms, which was diagnosed by computed tomography. The patient herein reported had a previous history of acute pancreatitis and had had a pancreatic pseudocyst treated by a cystogastrostomy 9 years earlier.
Received March 6th, 2011 - Accepted March 31st, 2011
Key words Pancreatic Pseudocyst; Pancreatitis
Disclosure There is no financial support or conflict of interest to declare by the authors
Correspondence
Carlos
M Nuño-Guzmán
Calle 68 No. 138. Sector Reforma
Guadalajara
Jalisco CP 44800
México
Phone: +133-3614.5501
Fax: +133-3609.6229
E-mail: carlosnunoguzman@hotmail.com
References
1. Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 1993:586-590. [PMID 8489394]
2. Rosso E, Alexakis N, Ghaneh P, Lombard M, Smart HL, Evans J, Neoptolemos JP. Pancreatic pseudocyst in chronic pancreatitis: endoscopic and surgical treatment. Dig Surg 2003; 20:397-406. [PMID 12900529]
3. Bradley EL, Clements JL Jr, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg 1979; 137:135-41. [PMID 758840]
4. Crass RA, Way LW. Acute and chronic pancreatic pseudocysts are different. Am J Surg 1981; 142:660-3. [PMID 7316029]
5. Sanfey H, Aguilar M, Jones RS. Pseudocysts of the pancreas, a review of 97 cases. Am Surg 1994; 60:661-8. [PMID 8060036]
6. Warshaw AL, Rattner DW. Timing of surgical drainage for pancreatic pseudocysts. Clinical and chemical criteria. Ann Surg 1985; 202:720-4. [PMID 4073984]
7. Lerch MM, Stier A, Wahnschaffe U, Mayerle J. Pancreatic pseudocysts: observation, endoscopic drainage, or resection? Dtsch Arztebl Int 2009; 106:614-21. [PMID 19890418]
8. Eisen GM, Chutkan R, Goldstein JL, Petersen BT, Ryan ME, Sherman S, et al. Endoscopic therapy of chronic pancreatitis. Gastrointest Endosc 2000; 52:843-8. [PMID 11182688]
9. Vignesh S, Brugge WR. Endoscopic diagnosis and treatment of pancreatic cysts. J Clin Gastroenterol 2008; 42:493-506. [PMID 18344889]
10. Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol 2009; 15:38-47. [PMID 19115466]
11. Shatney CH, Lillehei RC. Surgical treatment of pancreatic pseudocysts. Analysis of 119 cases. Ann Surg 1979; 189:386-94. [PMID 443893]
12. Spivak H, Galloway JR, Amerson JR, Fink AS, Branum GD, Redvanly RD, et al. Management of pancreatic pseudocysts. J Am Coll Surg 1998; 186:507-11. [PMID 9583690]
13. Bradley EL, Gonzalez AC, Clements JL Jr. Acute pancreatic pseudocyst: incidence and implications. Ann Surg 1976; 184:734-7. [PMID 999349]
14. Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology 1995; 108:1230-5. [PMID 7535275]
15. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126:1330-6. [PMID 15131794]
16. Degen L, Wiesner W, Berlinger C. Cystic and solid lesions of the pancreas. Best Pract Res Clin Gastroenterol 2008; 22:91-103. [PMID 18206815]
17. Gumaste VV, Aron J. Pseudocyst management. Endoscopic drainage and other emerging techniques. J Clin Gastroenterol 2010; 44:326-31. [PMID 20142757]
18. Aghdassi AA, Mayerle J, Kraft M, Sielenkämper AW, Heidecke CD, Lerch MM. Pancreatic pseudocysts. When and how to treat? HPB (Oxford) 2006; 8:432-41. [PMID 18333098]
19. Cannon JW, Callery MP, Vollmer CM Jr. Diagnosis and management of pancreatic pseudocysts: what is the evidence? J Am Coll Surg 2009; 209:385-93. [PMID 19717045]
20. Grewal HP, London NJM, Carr-Locke D, Wood KF. Endoscopic drainage of a recurrent pancreatic pseudocyst. Postgrad Med J 1990; 66:1081-1083. [PMID 2084661]
21. Struve CW. Spontaneous resolution of a pancreatic pseudocyst 10 years after detection documented by ultrasound. Pancreas 1993; 8:273-8. [PMID 8460102]