ORIGINAL ARTICLE
JOP. J Pancreas (Online) 2011 Jan 5; 12(1):19-25.
Bacteriology of Infection in Severe Acute Pancreatitis
Mohd T Noor1, Yellapu Radhakrishna1, Rakesh Kochhar1, Pallab Ray2, Jai Dev Wig3, Saroj K Sinha1, Kartar Singh1
Departments of 1Gastroenterology, 2Microbiology and 3General Surgery, Postgraduate Institute of Medical Education and Research. Chandigarh, India
ABSTRACT
Context Severe acute pancreatitis is associated with high mortality with infectious complications being the most common cause of mortality. Objective To analyze the prevalence and characteristics of pancreatic and extrapancreatic infection in patients with severe acute pancreatitis. Design Prospective study over a one-year period. Patients Fifty-one consecutive patients with severe acute pancreatitis. Setting Tertiary care centre, Northern India. Main outcome measures The presence of pancreatic and extrapancreatic infections were noted in consecutive patients with severe acute pancreatitis and their effect on disease outcome was assessed. Results Pancreatic infection was noted in 19 (37.3%) patients; 14 (27.5%) patients had monomicrobial and 5 (9.8%) patients had polymicrobial infections. In the first week of hospitalization, all positive 6/6 (100%) cultures grew Escherichia coli, in the second week 5/8 (62.5%) grew Escherichia coli while after the second week, 2/5 (40.0%) cultures grew Escherichia coli. A total of 32 (62.7%) patients had evidence of extrapancreatic infections, with 53 positive cultures. Fifteen (29.4%) patients had monomicrobial infections while 17 (33.3%) had polymicrobial infections. The most common site was blood together with intravenous site with 21 positive cultures in 16 patients. Staphylococcus aureus was most commonly isolated in the blood cultures. There was a statistically significant increase in mortality with pancreatic (P=0.003) and extrapancreatic (P=0.041) infections. The antibiotic sensitivity pattern showed that most of the bacteria were sensitive to beta lactum antibiotics, aminoglycosides and imipenem. Conclusion Pancreatic infections are more often monomicrobial with a shift from gram-negative to gram-positive as the pancreatitis progressed. Extrapancreatic infections are more often polymicrobial; most commonly, the blood stream is invaded by gram-positive bacteria.
INTRODUCTION
Acute pancreatitis is an acute inflammatory process of the pancreas with variable involvement of other regional tissues or remote organ systems. Infected pancreatic necrosis and pancreatic abscess are the most devastating of the complications leading to secondary pancreatic infections. Infectious complications are observed in 40-70% of all patients with severe acute pancreatitis [1, 2, 3]. Today most deaths related to acute pancreatitis occur after the first 7 to 10 days as a result of infective complications, particularly infected pancreatic necrosis [4, 5, 6, 7]. The infection rate correlates with the extent of the pancreatic necrosis and the bacterial contamination of the pancreatic necrosis, determines the course of the disease and patient outcome [5]. Pancreatic necrosis in the presence of severe or prolonged systemic complications, if sterile, has a mortality rate of 20% while, in the case of infected necrosis, it increases to more than 50% [8]. Infected necrosis is associated with a high incidence of organ failure irrespective of the extent of the necrosis [8]. There is evidence that the bacteria originate from the gastrointestinal tract [9]. These patients are often given prophylactic antibiotics but there are reports that the use of this strategy may result in the development of an infection with resistant bacteria or fungi [10].
Patients with severe pancreatitis often require prolonged hospitalization and multiple interventions. They often have extrapancreatic infections which may also influence the outcome. However, there are only a few studies in the literature regarding the occurrence of extrapancreatic infections and their microbiological spectrum. We conducted this study to evaluate the prevalence and characteristics of pancreatic and extrapancreatic infections in patients with severe acute pancreatitis and to determine their effect on patient outcome. An additional objective was to look at the sensitivity pattern of the cultured microorganisms.
MATERIAL AND METHODS
This study involved 51 consecutive patients (40 males, 11 females; mean age 41.8±15.3 years) with severe acute pancreatitis seen by us over a one-year period in the Department of Gastroenterology of the Postgraduate Institute of Medical Education and Research, a tertiary care referral centre at Chandigarh, India. Patients above eighteen years of age with their first attack of severe acute pancreatitis were included in the study. The diagnosis of acute pancreatitis was made based on clinical features, elevated serum amylase and/or lipase levels (more than 3-fold the upper reference limit) and evidence of pancreatitis on contrast-enhanced computed tomography (CECT) of the abdomen. Severe acute pancreatitis was diagnosed if the patient had organ failure according to the Atlanta criteria [11], the CT severity index (CTSI) greater than 6 [12], the APACHE-II score greater than 8 and, when available, the Ranson score greater than 3 [13, 14]. Patients with known chronic pancreatitis or previous surgery for pancreatic disease were excluded from the study.
Table 1 gives patient characteristics. The most common etiology was alcohol followed by gallstones and other causes. The APACHE II score ranged from 9-10 in 28 patients (54.9%), 11-20 in 20 patients (39.2%) and 21-30 in 3 patients (5.9%). The CTSI ranged from 7-10: 20 patients (39.2%) had a CTSI of 10, 10 patients (19.6%) had a CTSI of 9, 15 patients (29.4%) had a CTSI of 8, and 6 patients (11.8%) had CTSI of 7. The Ranson score could be calculated in only three patients as the majority of the patients presented more than 48 hours after the onset of the acute pancreatitis. It was 4 in two patients (66.7%) and 3 in one patient (33.3%) of the 51 patients with severe acute pancreatitis studied.
|
Table 1. Patient characteristics. |
|
|
Total number of patients |
51 |
|
Age; years (Mean±SD) |
41.8±15.3 |
|
Gender: |
|
|
Duration of hospitalization; days (Mean±SD) |
30.3±20.3 |
|
Etiology: |
|
|
CECT necrosis: |
|
|
CECT: contrast-enhanced computed tomography |
|
All patients underwent analysis of hematocrit, total and differential blood counts, liver function tests, blood urea nitrogen and serum creatinine, blood gas analysis, blood sugar, serum calcium, coagulation profile and imaging consisting of a chest X-ray, an abdominal X-ray, and ultrasound and CECT of the abdomen.
All patients were closely monitored for pancreatic and extrapancreatic infections. Samples were taken for culture from the blood, urine, throat, intravenous cannula tip, urinary catheter tip, tracheal aspirate (in those on a ventilator), drain fluid (if instituted) and bile (if drained). The culture media used were bile broth and trypticase-soy broth. Samples were taken at presentation, at 1 week, at 2 weeks and when the patient had a fever and/or leukocytosis. Pancreatic tissue was obtained either by ultrasound/CT guided fine needle aspiration (FNA) of the pancreatic necrotic tissue/peripancreatic collection or during surgery, and sent for bacteriological culture and gram staining in patients with suspected pancreatic infection. Tissue samples from patients undergoing necrosectomy were homogenized in a mechanical homogenizer before culture.
All patients were managed with prophylactic broad-spectrum antibiotics, parenteral/nasojejunal feeding, fluid and electrolyte management and supportive care with close monitoring. Patients admitted directly to the unit were given ciprofloxacin and metronidazole while those transferred from other hospitals continued the antibiotics they were already receiving. Indications for necrosectomy were: i) documented infected necrosis on culture, ii) worsening clinical condition and iii) persistent organ failure despite supportive care. Antibiotics were modified as per the sensitivity report. The patients were followed up until death or discharge from the hospital.
ETHICS
The protocol for the study was approved by the Ethical Committee of our institution, and written informed consent was obtained from all patients. The study protocol conforms to the ethical guidelines of the “World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, as revised in Tokyo 2004.
STATISTICAL ANALYSIS
The prevalence, spectrum and antibiotic sensitivity of bacterial infections were compiled and expressed as percentages. All quantitative variables were expressed as mean±SD. The association of pancreatic and extrapancreatic infections with the extent of the necrosis and clinical outcome was determined using the linear-by-linear chi-square test and the Fisher’s exact test when ordinal and dichotomic variables were involved, respectively. The frequencies of pancreatic and extrapancreatic infections were compared by means of the McNemar test. A two-tailed P value of less than 0.05 was considered significant. Statistical analysis was carried out using the statistical software package SPSS version 17.0 (SPSS, Chicago, Illinois, USA).
RESULTS
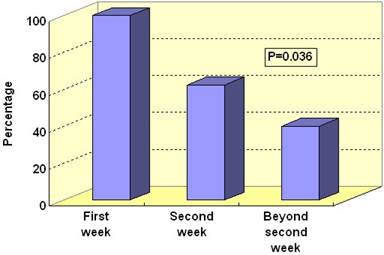
Pancreatic infection was documented in 19 (37.3%) out of the 51 patients. The fine needle aspirate taken from the pancreatic tissue was positive in 14 patients and the culture of pancreatic tissue obtained at surgery was positive in 8 patients, with both being positive in 3 patients. Of the 22 positive pancreatic cultures, 19 (86.4%) grew gram-negative organisms while only 3 (13.6%) grew gram-positive organisms. All patients having both a positive fine needle aspirate culture and a positive culture of the operative tissue had identical organisms. Fourteen (73.7%) of the 19 patients had monomicrobial and 5 patients (26.3%) had polymicrobial infections. Table 2 gives a list of organisms cultured from the pancreatic tissue. There was a positive significant association between pancreatic infection and the degree of pancreatic necrosis: pancreatic infection was present in 1/6 (16.7%) patients with <30% necrosis, in 4/20 (20.0%) patients with 30-50% necrosis and in 14/25 (56.0%) patients with >50% necrosis (P=0.013). Of the 19 patients with pancreatic infection, 6 (31.6%) had infection in the first week of hospitalization, 8 (42.1%) in the second week and 5 (26.3%) in the third week or later. In the first week of hospitalization, all positive 6/6 (100%) cultures grew Escherichia coli, 5/8 (62.5%) grew Escherichia coli in the second week while 2/5 (40.0%) cultures were positive for Escherichia coli after the second week (P=0.036; Figure 1).
|
Table 2. Microorganisms isolated in pancreatic and extrapancreatic infections in 51 patients with severe acute pancreatitis. |
||
|
|
Pancreatic |
Extrapancreatic |
|
Total positive cultures |
22 |
53 |
|
Escherichia coli |
13 (59.1%) |
11 (20.8%) |
|
Staphylococcus aureus |
1 (4.5%) |
10 (18.9%) |
|
Enterococcus faecalis |
2 (9.1%) |
9 (17.0%) |
|
Acinetobacter baumannii |
1 (4.5%) |
9 (17.0%) |
|
Pseudomonas aeruginosa |
3 (13.6%) |
4 (7.5%) |
|
Klebsiella pneumoniae |
2 (9.1%) |
2 (3.8%) |
|
Enterobacter aerogenes |
0 |
3 (5.7%) |
|
Alcaligenes xylosoxidans |
0 |
3 (5.7%) |
|
Morganella morganii |
0 |
1 (1.9%) |
|
Proteus mirabilis |
0 |
1 (1.9%) |
|
Figure 1. Proportion of infections caused by Escherichia coli out of all bacterial infections in 51 patients with severe acute pancreatitis. Data are given in percentages. |
A total of 32 (62.7%) patients had evidence of extrapancreatic infections, with 53 positive cultures. The number of extrapancreatic infections was significantly higher than pancreatic infections (P=0.021) and there was no association between the presence of the two infections (P=1.000); in fact, 12 (23.5%) cases had no infection; 12 (23.5%) cases had both infections; 7 (13.7%) cases had pancreatic infections only; and 20 cases had extrapancreatic infections only. Of the 53 positive cultures, 19 (35.8%) were present in the first week, 9 (17.0%) in the second week and 25 (47.2%) after the second week. Fifteen of the 32 patients (46.9%) had monomicrobial infections while 17 (53.1%) had polymicrobial extrapancreatic infections. Table 3 gives the source of the 53 positive cultures. The most common site of extrapancreatic infection was the blood together with the intravenous site with 21 cultures (39.6%) in 16 patients; eight (38.1%) of these 21 cultures were positive in the first week, 5 (23.8%) in the second week and 8 (38.1%) after the second week. Overall, 37 (69.8%) organisms were gram-negative and 16 (30.2%) were gram-positive.
|
Table 3. Sites of 53 extrapancreatic infections found in 51 patients with severe acute pancreatitis. |
|
|
Site |
No. of cases (%) |
|
Blood culture |
16 (30.2%) |
|
Intravenous site culture |
5 (9.4%) |
|
Urine culture |
7 (13.2%) |
|
Tracheal aspirate culture |
5 (9.4%) |
|
Bile fluid culture |
2 (3.8%) |
|
Ascitic fluid culture |
3 (5.7%) |
|
Pleural fluid culture |
3 (5.7%) |
|
Drain fluid culture |
12 (22.6%) |
Twenty-one organisms were isolated in patients with a positive blood culture: Staphylococcus aureus was the most common organism (n=6; 28.6%) followed by Escherichia coli (n=4; 19.0%), Enterobacter aerogenes (n=3; 14.3%), Alcaligenes xylosoxidans (n=2; 9.5%), Enterococcus faecalis (n=2; 9.5%), Pseudomonas aeruginosa (n=2; 9.5%), Acinetobacter baumannii (n=1; 4.8%), and Klebsiella pneumoniae (n=1; 4.8%). Gram-negative bacilli were most commonly isolated in patients with a positive nasobiliary drain culture, pleural fluid culture and abdominal drain fluid culture (Table 4).
|
Table 4. Microorganisms isolated in pancreatic and extrapancreatic infections in 51 patients with severe acute pancreatitis. |
||
|
Site |
No of micro-organisms |
Organism grown |
|
Intraoperative specimen |
8 |
Escherichia coli (4), Enterococcus faecalis (1), Pseudomonas aeruginosa (2), Klebsiella pneumoniae (1) |
|
US/CT aspirate |
14 |
Escherichia coli (8), Enterococcus
faecalis (1), Pseudomonas aeruginosa (1), Klebsiella pneumoniae
(1), |
|
Blood |
21 |
Staphylococcus aureus (6), Escherichia coli (4), Enterobacter aerogenes (3), Alcaligenes xylosoxidans (2), Enterococcus faecalis (2), Pseudomonas aeruginosa (2), Acinetobacter baumannii (1), Klebsiella pneumoniae (1) |
|
Intravenous site |
5 |
Enterococcus faecalis (1), Klebsiella pneumoniae (1), Staphylococcus aureus (2), Acinetobacter baumannii (1) |
|
Urine |
7 |
Escherichia coli (3), Enterococcus faecalis (1), Yeast (3) a |
|
Tracheal aspirate |
5 |
Acinetobacter baumannii (3), Morganella morganii (1), Enterococcus faecalis (1) |
|
NBD fluid |
3 |
Escherichia coli (1), Enterococcus faecalis (1), Pseudomonas aeruginosa (1) |
|
Ascitic fluid |
3 |
Acinetobacter baumannii (1), Enterococcus faecalis (2) |
|
Pleural fluid |
3 |
Acinetobacter baumannii (2), Escherichia coli (1) |
|
Drain fluid |
12 |
Escherichia coli (3), Acinetobacter baumannii (1), Alcaligenes xylosoxidans (1), Staphylococcus aureus (1), Enterococcus faecalis (1), Pseudomonas aeruginosa (1), Proteus mirabilis (1), Yeast (3) a |
|
a Yeast was not taken into consideration in the list of microorganisms in Tables 2 and 5 in which only bacteria were listed. Since some of the infections were polymicrobial, the total type of organisms are more than the number of infected sites. CT : computed tomography; NBD: nasobiliary drain; US : ultrasound |
||
Table 5 gives the sensitivity pattern of organisms isolated from the pancreatic tissue and the extrapancreatic sites of the 51 patients. The antibiotic sensitivity pattern showed that the majority of the bacteria were sensitive to beta lactum antibiotics, aminoglycosides and imipenem.
|
Table 5. Antibiotic sensitivity pattern of the pathogens cultured. |
|
|
Organism |
Antibiotic sensitivity |
|
Escherichia coli |
Amikacin (17;
70.8%), Imipenem (8; 33.3%), Pipercillin+tazobactum (7; 29.2%), Netilmicin
(4; 16.7%), |
|
Staphylococcus aureus |
Amikacin
(4; 36.4%), Netilmicin (3; 27.3%), Ciprofloxacin (3; 27.3%), Methicillin (3;
27.3%), |
|
Enterococcus faecalis |
Vancomycin (5;
45.5%), Pipercillin+tazobactum (3; 27.3%), Penicillin (1; 9.1%), Amoxicillin
(3; 27.3%), |
|
Acinetobacter baumannii |
Cefoperazone +
sulbactam (5; 50.0%), Imipenem (4; 40.0%), Pipercillin+tazobactum (4; 40.0%), |
|
Pseudomonas aeruginosa |
Imipenem
(4; 57.1%), Amikacin (4; 57.1%), Pipercillin+tazobactum (3; 42.9%),
Netilmicin (2; 28.6%), |
|
Klebsiella pneumoniae |
Imipenem (3;
75.0%), Pipercillin+tazobactum (2; 50.0%), Ciprofloxacin (2; 50.0%), |
|
Enterobacter aerogenes |
Pipercillin+tazobactum
(2; 66.7%), Netilmicin (1; 33.3%), Cefoperazone + sulbactam (1; 33.3%), |
|
Alcaligenes xylosoxidans |
Cotrimoxazole (2; 66.7%), Ciprofloxacin (1; 33.3%), Gentamicin (1; 33.3%), Netilmicin (1; 33.3%), Amikacin (1; 33.3%) |
|
Morganella morganii |
Imipenem (1; 100%), Amikacin (1; 100%) |
|
Proteus mirabilis |
Imipenem (1; 100%) |
Out of 51 patients, 29 (56.9%) were managed conservatively while 22 (43.1%) underwent surgery. Of these, 15 (68.2%) underwent an exploratory laparotomy and pancreatic necrosectomy whereas 7 (31.8%) underwent an exploratory laparotomy, pancreatic necrosectomy and lesser sac drainage. In 9 (40.9%) of these 22 patients, percutaneous drainage had already been carried out. Surgery was performed on them due to further deterioration despite drainage. In the rest of the patients (n=13; 59.1%), the clinical decision for surgery was taken as per the indications specified in the “Material and Methods” section.
A total of 29 patients died (56.9%). Patients with pancreatic infections had a higher mortality rate as compared to patients without pancreatic infections (16/19, 84.2% vs. 13/32, 40.6%, P=0.003). Mortality was higher in patients with extrapancreatic infections as compared to patients without extra-pancreatic infections (22/32, 68.8% vs. 7/19, 36.8%; P=0.041). Of the 29 patients who died 16 (55.2%) had undergone surgery, while, of the 22 patients who survived, only 6 (27.3%) had undergone surgery. The mortality rate was higher in patients who underwent surgery(59.1% vs. 44.8%) but did not reach statistical significance (P=0.086).
DISCUSSION
In the present study, we investigated pancreatic and extrapancreatic infections in patients with severe acute pancreatitis. Pancreatic infections were observed in 19 (37.3%) patients, with Escherichia coli being the most common organism. There was a change in the spectrum of organisms as the pancreatitis progresses, which changed from predominantly gram-negative to gram-positive organisms. Extrapancreatic infections were seen in 32 (62.7%) patients and were more often polymicrobial as compared to pancreatic infections (53.1% vs. 26.3%; P=0.083). We have studied the characteristics and impact of pancreatic and extrapancreatic infections on the clinical course of these patients separately.
In accordance with the reports of other studies [15, 16], the prevalence of the pancreatic infections correlated with the extent of the necrosis. There was a shift from gram-negative to gram-positive organisms with increase in length of hospital stay. The most common mechanism involved in the pathogenesis of the pancreatic infections was the translocation of bacteria from the gut [17, 18]. This could explain pancreatic infection with Escherichia coli, which is reported to be the most common organism in this setting [19]. Subsequent infection with gram-positive organisms could be secondary to nosocomial blood stream invasion. In patients with pancreatic infections, monomicrobial infections were more common than polymicrobial infections. Similar findings have been reported in other studies in the literature [19]. Polymicrobial infections are more common in patients with pancreatic abscess as compared to patients with infected pancreatic necrosis [20]. Most of the other studies in the literature have also found Escherichia coli to be the most common isolate [2, 19, 21]. However, Gerzof et al. [3] reported Klebsiella to be the most common isolate.
Extrapancreatic infections have not been the focus of attention of many studies. We had 53 positive cultures in 32 patients with 19 cultures being positive in the first week, 9 in the second week and 25 in the third week and later. There was no correlation between the occurrence of pancreatic and extrapancreatic infections or the organisms grown at the two sites. The most common extrapancreatic site involved was the blood stream with positivity documented in 16 (30.2%) cultures followed by drain fluid culture positivity in 12 (23.5%) patients. While Escherichia coli was the most common organism in urine and drain fluid, Staphylococcus aureus was the most common organism in the blood and Acinetobacter baumannii in the tracheal aspirate.
The timing and correlation of extrapancreatic infections with pancreatic infections in patients with acute pancreatitis has previously been investigated only in a few studies. In an animal model of acute pancreatitis involving 65 rats, extrapancreatic bacterial infections were investigated in the spleen, liver, mesenteric lymph nodes, peritoneal cavity and blood. The animals were sacrificed at 8, 16, 24 and 32 hours after the induction of acute pancreatitis. The peak of bacterial culture positivity was seen at 16-24 hours in the spleen and liver. There was no bacterial growth in the mesenteric lymph nodes in the 8-hour group. However, after 16, 24, and 32 hours, 46.2%, 76.9%, and 38.5% of the cultures were positive, respectively. Bacteria could be cultured from the peritoneal cavity in 53.9% of the control group and in 76.9 to 92.3% of the animals with pancreatitis [9]. In a study of 212 patients with acute pancreatitis, Bourgaux et al. [22] reported extrapancreatic infections in 25% of their patients. The most common sites of infection were the peritoneal fluid (26.8%), blood (24.4%), respiratory tract (24.4%) and urinary tract (19.5%). Infections were polymicrobial in 37.5% of patients. The median time between the onset of the pancreatitis and the diagnosis of the infection was 4 days; it was shorter than that of pancreatic infections.
In a study by Garg et al. [19], extrapancreatic bacterial infections were found in 31.7% of the 63 patients. Acute cholangitis occurred in 6 patients, intravenous site infection in 5 patients, and urine and peritoneal fluid infection occurred in 3 patients each. The most common organisms isolated were Escherichia coli in 25% of the cultures and Pseudomonas aeruginosa in 23%of the cultures. In a recent study by Besselink et al. [23], bacteremia was reported in 13.4% and pneumonia in 11.5% of patients with their first episode of acute pancreatitis. Bacteremia and pneumonia were diagnosed earlier than infected necrosis. They found gram-negative bacteria to be the most common isolates. However, a tendency towards an increase in gram-positive bacteria has been noted in several series [1, 10, 24]. This might be explained by the use of prophylactic antibiotic therapy against gram-negative bacteria [10].
Differences in the types of microorganisms between pancreatic and extrapancreatic infections may reflect the different sources of infection. While the predominant gram-negative bacteria in pancreatic infections are derived from the endogenous bowel microflora [25], the source of predominant gram-positive bacteria in extrapancreatic infections is probably exogenous nosocomial. Factors which predispose these patients to infections include the use of intravascular, percutaneous and urinary catheters, mechanical ventilation and surgical intervention. Willdison et al. [26] showed a higher incidence of bacteremia in patients with pancreatic infections. The source of bacteremia was respiratory (17%), genitourinary (17%), biliary (3%), skin (3%) and the intravenous site (3%).
The presence of pancreatic infections adversely affected the outcome of these patients with mortality being significantly higher in patients with pancreatic infections. Surgical intervention was another factor which adversely affected the outcome of these patients with mortality being significantly higher in patients who underwent surgery. We also found higher mortality rates in patients with extrapancreatic infections. In another study [23], patients with infected pancreatic necrosis had a 2.5 times higher mortality rate if bacteremia or pneumonia was documented. Bacteremia is a risk factor for infection of pancreatic necrosis; the cultured pathogens point to the gut as the source of the pathogen [23]. The presence of bacteremia should raise the level of suspicion for infected necrosis in a patient already diagnosed with pancreatic necrosis.
The antibiotic sensitivity pattern of our patients showed that most of the organisms were sensitive to beta lactum antibiotics, aminoglycosides and imipenem. In a study of incidence, spectrum and antibiotic sensitivity pattern of bacterial infections among patients with acute pancreatitis, Garg et al. [19] noted that most bacterial isolates were sensitive to third generation cephalosporins and quinolones. Imipenem, cephalosporins and quinolones are the antibiotics which achieve high pancreatic tissue concentration [27, 28, 29, 30].
Our study has a few limitations. We have not studied anaerobic infections, although they have occasionally been found in patients with severe acute pancreatitis [3, 22]. Our study included only patients with severe acute pancreatitis; bacterial infections in patients with milder grades of pancreatitis were therefore not studied. Most of our patients were referred; they had already received treatment at primary and secondary level hospitals, details of which could not be ascertained. We have not classified the infections into primary and secondary because many of the patients had already undergone interventions in other institutions before admission to our centre.
In conclusion, we observed pancreatic infections in 37.3% of patients and extrapancreatic infections in 62.7% of patients with severe acute pancreatitis. The pancreatic infections were most often monomicrobial while the extrapancreatic infections were more often polymicrobial. In patients with pancreatic infection, there was a change in the spectrum of the micro-organisms from gram-negative to gram-positive with increase in the progression of the disease. The most common site of the extrapancreatic infections was blood stream invasion. The present study showed that the presence of pancreatic as well as extrapancreatic infections adversely affected the outcome of patients with severe acute pancreatitis. In addition to pancreatic infections, early detection and treatment of extrapancreatic infections may positively affect patient outcome. The effect of the treatment of extrapancreatic infections on patient outcome should be investigated in future studies. The results of the sensitivity pattern of the microorganisms suggest that cephalosporins and imipenem should be used as the empirical antibiotics of choice in patients with severe acute pancreatitis.
Received September 22nd, - Accepted November 12th
Key words Pancreatitis, Acute Necrotizing; Bacterial Infections; Bacteremia
Abbreviations CTSI: CT severity index
Conflict of interest The authors have no potential conflict of interest
Correspondence
Rakesh Kochhar
Department of Gastroenterology
Postgraduate
Institute of Medical Education and Research
Chandigarh
India 160012
Phone: +91-172.275.6608
Fax: +91-172.274.4401
E-mail: dr_kochhar@hotmail.com
References
1. Gloor B, Müller CA, Worni M, Stahel PF, Redaelli C, Uhl W, et al. Pancreatic infection in severe pancreatitis: the role of fungus and multiresistant organisms. Arch Surg 2001; 136:592-6. [PMID 11343553]
2. Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis: a prospective clinical study. Gastroenterology 1986; 91:433-8. [PMID 3522342]
3. Gerzof SG, Banks PA, Robbins AH, Johnson WC, Spechler SJ, Wetzner SM, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology 1987; 93:1315-20. [PMID 3678750]
4. Schmid SW, Uhl W, Friess H, Malfertheiner P, Buchler MW. The role of infection in acute pancreatitis. Gut 1999; 45:311-6. [PMID 10403749]
5. Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br J Surg 1999; 86:1020-4. [PMID 10460637]
6. Allardyce D. Incidence of necrotizing pancreatitis and factors related to mortality. Am J Surg 1987; 154:295-99. [PMID 3631408]
7. de Beaux ac, Palmer KR, Carter DC. Factors influencing morbidity and mortality in acute pancreatitis: an analysis of 279 cases. Gut 1995; 37:121-6. [PMID 7672660]
8. Garg PK, Madan K, Pande GK, Khanna S, Sathyanarayan G, Bohidar NP, Tandon RK. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Clin Gastroenterol Hepatol 2005; 3:159-66. [PMID 15704050]
9. Schwarz M, Thomsen J, Meyer H, Büchler MW, Beger HG. Frequency and time course of pancreatic and extrapancreatic bacterial infection in experimental acute pancreatitis in rats. Surgery 2000; 127:427-32. [PMID 10776434]
10. Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg 2000; 232:619-26. [PMID 11066131]
11. Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 128:586-90. [PMID 8489394]
12. Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology 1990; 174:331-6. [PMID 2296641]
13. Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: A physiologically based classification system. Crit Care Med 1981; 9:591-7. [PMID 7261642]
14. Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974; 139:69-81. [PMID 4834279]
15. Bradley EL 3rd, Allen K. A prospective longitudinal study of observation versus surgical intervention in the management of necrotizing pancreatitis. Am J Surg 1991; 161:19-25. [PMID 1987854]
16. Vesentini S, Bassi C, Talamini G, Cavallini G, Campedelli A, Pederzoli P. Prospective comparison of C-reactive protein level, Ranson score and contrast-enhanced computed tomography in the prediction of septic complications of acute pancreatitis. Br J Surg 1993; 80:755-7. [PMID 8330167]
17. Liu H, Li W, Wang X, Li J, Yu W. Early gut mucosal dysfunction in patients with acute pancreatitis. Pancreas 2008; 36:192-6. [PMID 18376312]
18. Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979; 23:403-11. [PMID 154474]
19. Garg PK, Khanna S, Bohidar NP, Kapil A, Tandon RK. Incidence, spectrum and antibiotic sensitivity pattern of bacterial infections among patients with acute pancreatitis. J Gastroenterol Hepatol 2001; 16:1055-9. [PMID 11595073]
20. Bittner R, Block S, Büchler M, Beger HG. Pancreatic abscess and infected pancreatic necrosis. Different local septic complications in acute pancreatitis. Dig Dis Sci 1987; 32:1082-7. [PMID 3308374]
21. Bradley EL 3rd. A fifteen year experience with open drainage for infected pancreatic necrosis. Surg Gynecol Obstet 1993; 177:215-22. [PMID 8356492]
22. Bourgaux JF, Defez C, Muller L, Vivancos J, Prudhomme M, Navarro F, et al. Infectious complications, prognostic factors and assessment of anti-infectious management of 212 consecutive patients with acute pancreatitis. Gastroenterol Clin Biol 2007; 31:431-5. [PMID 17483784]
23. Besselink MG, van Santvoort HC, Boermeester MA, Nieuwenhuijs VB, van Goor H, Dejong CH, et al. Timing and impact of infections in acute pancreatitis. Br J Surg 2009; 96:267-73. [PMID 19125434]
24. Luiten EJ, Hop WC, Lange JF, Bruining HA. Differential prognosis of gram-negative versus gram-positive infected and sterile pancreatic necrosis: results of a randomized trial in patients with severe acute pancreatitis treated with adjuvant selective decontamination. Clin Infect Dis 1997; 25:811-6. [PMID 9356793]
25. Gianotti L, Munda R, Alexander JW, Tchervenkov JI, Babcock GF. Bacterial translocation: a potential source for infection in acute pancreatitis. Pancreas 1993; 8:551-8. [PMID 8302791]
26. Widdison AL, Karanjia ND. Pancreatic infection complicating acute pancreatitis. Br J Surg 1993; 80:148-54. [PMID 8443638]
27. Büchler M, Malfertheiner P, Friess H, Isenmann R, Vanek E, Grimm H, et al. Human pancreatic tissue concentration of bactericidal antibiotics. Gastroenterology 1992; 103:1902-8. [PMID 1451983]
28. Spicák J, Martínek J, Závada F, Morávek J, Melenovsky V. Penetration of antibiotics into the pancreas in rats: an effect of acute necrotizing pancreatitis. Scand J Gastroenterol 1999; 34:92-7. [PMID 10048739]
29. Gloor B, Worni M, Strobel O, Uhl W, Tcholakov O, Müller CA, et al. Cefepime tissue penetration in experimental acute pancreatitis. Pancreas 2003; 26:117-21. [PMID 12604907]
30. Papagoras D, Giamarellos-Bourboulis EJ, Kanara M, Douridas G, Paraskevopoulos I, Antzaklis G,et al. Pancreatic concentrations of cefepime in experimental necrotizing pancreatitis. J Chemother 2003; 15:43-6. [PMID 12678413]