ORIGINAL ARTICLE
JOP. J Pancreas (Online) 2000; 1(3):49-57.
Effect of Protein
Kinase C on Glucose-Mediated Insulin Secretion in HIT-T15 Cells
Hiroko Akiyoshi, Yutaka Nakaya
Department of Nutrition, School of Medicine, The
University of Tokushima. Tokushima City, Japan
ABSTRACT
Objective To clarify
the regulation of protein kinase C on glucose-mediated insulin secretion. Main
outcome measures We examined the effect of protein kinase C on the
cytosolic free Ca2+ concentration ([Ca2+]i)
and the activity of Ca2+-activated K+ channels (KCa-channel)
in the insulinoma cell line, HIT-T15. Results Glucose at a concentration
of 10 mmol/L increased the secretion of insulin. This increase was partly
inhibited by 1 nmol/L staurosporine, a protein kinase C inhibitor.
Staurosporine (1 nmol/L) also attenuated the glucose-induced elevations in [Ca2+]i.
On the contrary, glibenclamide (100 nmol/L) specifically blocked ATP-sensitive
K+ channels, and increased both [Ca2+]i and
insulin secretion, but staurosporine had no effect on them. Patch clamp studies
showed that 10 mmol/L glucose almost completely blocked KCa channel
activity, an effect that was reversed by 1 nmol/L staurosporine. Phorbol
12-myristate 13-acetate (1 mmol/L), a protein kinase C activator, also decreased
KCa channel activity. Conclusions These results indicate that
the activation of protein kinase C is involved in the glucose-induced release
of insulin by modulating K+ channel function in HIT-T15 cells.
INTRODUCTION
The physiological secretagogue, glucose, causes
insulin to be released by pancreatic beta cells through a complex mechanism.
This process involves the closure of ATP-sensitive K+ (KATP)
channels [1], which are directly blocked by intracellular ATP [2], membrane
depolarization [3], the opening of voltage-gated L-type Ca2+
channels, and a subsequent increase in the cytosolic Ca2+
concentration ([Ca2+]i) [4]. Thus, the closure of KATP
channels is regarded as a prerequisite for the glucose-induced insulin release.
Sulfonylureas, used in the treatment of diabetes mellitus, have been shown to
specifically block the KATP channel [5], thus inducing cell membrane
depolarization and insulin secretion. The opposite has been found for the
hyperglycemic drug diazoxide, a compound which opens KATP channels,
hyperpolarizes the beta cells and inhibits the glucose-induced release of
insulin in rat islets [6].
Several reports have shown that glucose can cause the
beta-cells to release insulin without altering the cell’s membrane potential or
[Ca2+]i level [7, 8, 9]. In addition to the block of the
KATP channels, insulin release is regulated by a variety of
intracellular processes, including changes in intracellular levels of cyclic
nucleotides and phosphoinositide turnover. The latter process is mediated by
the activation of phospholipase C, resulting in the generation of inositol 1,
4, 5 trisphoshate and diacylglycerol [10]. Whereas inositol 1, 4, 5
trisphoshate releases intracellularly bound Ca2+ [11],
diacylglycerol exerts its effects through the activation of protein kinase C
(PKC) [12]. The activity of this enzyme has been established in both rat [13]
and mouse [14] pancreatic islets as well as in insulin-producing tumor cells [15,
16]. In HIT-T15 cells, Deeney et al. [17] reported that glucose caused
translocation of PKC from the cytoplasm to the plasma membrane. Several types
of ion channels have also been shown to be modulated by the activation of PKC [18].
For example, phorbol esters and synthetic diacylglycerol, which activate PKC,
reportedly block K+ currents [19]. In addition to KATP
channels, several other K+ channels were characterized in cultured pancreatic
islet cells [20]. However, it is unclear how the individual K+ channel
currents are affected by PKC in pancreatic beta cells. Therefore, we attempted
to examine the effects of PKC on glucose-induced insulin secretion via KCa
channels by using a PKC inhibitor, staurosporine, in insulinoma HIT-T15 cells.
MATERIALS AND METHODS
Cell Culture
The insulin secreting cell line, HIT-T15 (Dainippon
Pharmaceutical Co., Ltd, Osaka, Japan), was cultured at 37 °C in Ham’s F12-K
medium (Dainippon Pharmaceutical Co., Ltd, Osaka, Japan) supplemented with 10%
fetal bovine serum, 100 IU/ml penicillin and 100 mg/ml streptomycin (all
obtained from GIBCO, Grand Island, USA) in an atmosphere of 5% CO2.
The medium was changed twice a week and the cell line was passaged once a week.
Measurements of Insulin
Secretion
HIT cells were plated into 24-well tissue culture
dishes (1 to 10 x 105 cells/well). Prior to any analysis, the plates
were rinsed with Tyrode’s solution. This solution contained 128 mmol/L NaCl,
2.68 mmol/L KCl, 1.8 mmol/L CaCl2, 1.64 mmol/L MgCl2 and
10 mmol/L MOPS (3-[N-morpholinol] propane-sulfonic acid), pH 7.2. Subsequently,
the plates were incubated with test reagents diluted in the same buffer for 60
min. The samples were then collected and frozen at -20 °C. Insulin was measured
by radioimmunoassay with commercially available kits according to the
manufacturer’s specifications (Waco, Tokyo, Japan).
Measurement of Intracellular Ca2+
The [Ca2+]i levels were studied
by using the fluorescent dye fura-2AM (Waco, Tokyo, Japan). Cells were cultured
on glass coverslips three days before the experiments. At the time of analysis,
these cells were immersed in Tyrode’s solution containing 4 mmol/L fura-2AM for
30 min at 37 °C. The cells were then transferred to a small incubation bath
(0.5 mL) that had been mounted on a microscope stage. The bath temperature was
maintained at 37 °C while being perfused with buffer at a rate of 0.8 mL/min
during the experimental period. Any fluorescence emitted was measured in the
HIT cells on the coverslips by using a fluorescence spectromicroscope
(excitation, 340/380 nm, emission, 510 nm). The results were expressed as a
ratio of the fluorescence recorded at 340 nm and 380 nm.
Electrophysiologic Measurements
Membrane currents were recorded in the
"cell-attached" and "inside-out" configurations by
employing a patch-clamp amplifier as described by Hamill et al. [21].
The bath solution for the cell-attached mode contained 140 mmol/L KCl, 10
mmol/L MOPS-K, and 1 mmol/L CaCl2. The pipette solution was the same
as the cell-attached solution except for the Ca2+ concentration
(CaCl2 10-7 mol/L). Soft glass pipettes, prepared in an
electrode puller (PP-83, Narishige, Tokyo, Japan), were used after being coated
with Sylgard. The electrical resistance of the patch pipette was 5 to 7 MW for
single channel recording. Experiments were conducted at a solution temperature
between 35 and 37 °C. Data were stored in a PCM recorder (model PCM-501ES, Sony
Co., Tokyo, Japan) with a low pass filter (3 KHz). The pClamp Ver 6.0 software
(Axon Instruments Inc., Foster City, USA) was used to analyze the data on
single channel currents. The open probability (NPo) was determined from current
amplitude histograms and was calculated by using the following equation:
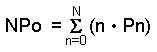
where N is the number of channels in the patch and Pn
is the integrated channel opening.
STATISTICAL ANALYSIS
Data are expressed as mean±SD. Differences among data
sets were evaluated by the Student’s t-test. A level of P<0.05 was accepted
as statistically significant.
RESULTS
Table 1 shows the effect of glucose on insulin
secretion in cultured HIT cells. Glucose (3, 10, 30 mmol/L) caused insulin
secretion in a concentration-dependent manner. To determine whether the effects
of glucose on insulin secretion involved a PKC, we studied the effect of a PKC
inhibitor, staurosporine, on insulin release. The glucose-induced secretion of
insulin (at the concentration of 10 mmol/L: 455±182 pmol/L/h, n=8) was
significantly attenuated in the presence of 1 nmol/L staurosporine (286±34
pmol/L/h, n=6, P<0.05 vs. 10 mmol/L glucose). In addition, phorbol
12-myristate 13-acetate (PMA: 1 mmol/L), an activator of PKC, alone
significantly increased insulin secretion.
|
Table 1. Effect of staurosporine on insulin secretion induced by glucose. |
|
|
|
Insulin release |
|
Control |
129±16 |
|
Glucose (3 mmol/L) |
268± 95 a |
|
Glucose (10 mmol/L) |
455±182 ac |
|
Glucose (30 mmol/L) |
497± 254 b |
|
Glucose (10 mmol/L) + Staurosporine (1
nmol/L) |
286±34 c |
|
Phorbol 12-myristate 13-acetate (PMA 1
mmol/L) |
280± 157 b |
|
Results are shown as mean values ± SD |
|
As shown in Table 2, glibenclamide stimulated insulin
secretion in a concentration-dependent manner between 10-1,000 nmol/L. Unlike
glucose, staurosporine did not attenuate the glibenclamide-induced insulin
secretion when stimulated with 100 nmol/L glibenclamide.
|
Table 2. Effect of staurosporine on insulin secretion induced by
glibenclamide. |
|
|
|
Insulin release |
|
Control |
129± 16 |
|
Glibenclamide (10 nmol/L) |
554± 173 a |
|
Glibenclamide (100 nmol/L) |
650± 268 a |
|
Glibenclamide (1000 nmol/L) |
662± 192 a |
|
Glibenclamide (100 nmol/L) +
Staurosporine (1 nmol/L) |
606± 220 |
|
Results are shown as mean values ± SD |
|
Glucose and glibenclamide increased the [Ca2+]i
level indicated by 340/380 ratio in a concentration-dependent manner (Figure
1). The 340/380 ratio was 0.928±0.199 in the resting conditions and the peak
elevation by 10 mmol/L glucose was 1.307±0.222 (P<0.001, n=8). To clarify
whether the decrease in insulin secretion caused by staurosporine was related
to changes in the [Ca2+]i, we tested the effect of
staurosporine on glucose-induced [Ca2+]i increases.
Staurosporine (1 nmol/L) significantly reduced the level of intracellular
calcium in the presence of glucose, (340/380 ratio; 1.307±0.222 to 1.123±0.180,
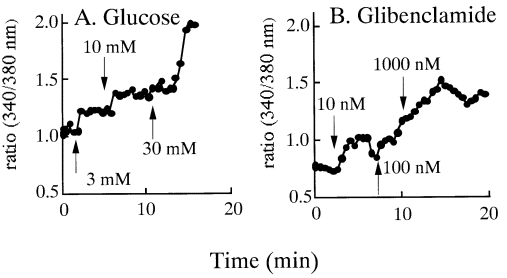
P<0.05, n=8, Figure 2A) and the time point was 16 min. PMA alone also
increased the [Ca2+]i (1.036±0.122 to 1.186±0.185,
P<0.001, n=11) and the time point was 3 min (Figure 2B).
|
Figure 1. The effects of glucose and glibenclamide on cytosolic Ca2+
in HIT cells. A. Glucose (3, 10, 30 mmol/L) and B.
Glibenclamide (10, 100, 1,000 nmol/L) were perfused. The results are
presented as the 340/380 nm wavelength ratio of fluorescence. |
|
Figure 2. A. Effect of staurosporine on glucose-induced [Ca2+]i
increase in HIT-T15 cells. Note that staurosporine partially suppressed
increase in [Ca2+]i by glucose. B. Effect of
phorbol 12-myristate 13-acetate (PMA) on [Ca2+]i
in fura-2 loaded HIT cells. There was a slight increase in [Ca2+]i
by PMA. C. Effect of staurosporine on glibenclamide induced on [Ca2+]i
in fura-2 loaded HIT cells. The increase in glibenclamide-induced [Ca2+]i
, and staurosporine and glibenclamide-induced [Ca2+]i
increase was almost at the same level. |
Glibenclamide at a concentration of 100 nmol/L
increased [Ca2+]i from a control level of 0.968±0.229 to
a peak level of 1.407± 0.137 (P<0.05, n=5), but staurosporine had no effect
on the glibenclamide-induced [Ca2+]i increase
(1.305±0.187, n=5, Figure 2C). These values were obtained after 9 min
application of drugs.
To investigate whether the decrease in insulin
secretion and [Ca2+]i levels caused by staurosporine were
related to the activity of the K+ channels, we studied single
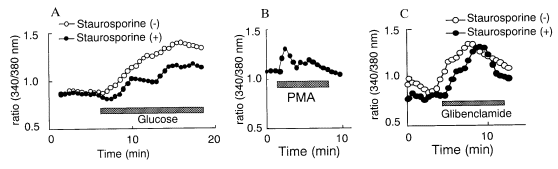
channel K+ currents using patch clamp technique. Figure 3A shows the
effect of glibenclamide on KATP (channels that had a conductance of
51±13 pS), (n=9, Figure 3A) and KCa channels (223±35 pS), (n=7,
Figure 3B). Glibenclamide (100 nmol/L) blocked KATP channel (Figure
3A), but had no effect of KCa channel (Figure 3B). The open
probability of the KATP channel was decreased from 0.154±0.088 to
0.014±0.025 (P<0.05, n=6). Figure 3C shows the effect of 10 mmol/L glucose
on K+ channel currents in the cell-attached configurations, using
symmetrical 150 mmol/L K+, without glucose and at a membrane
potential of +40 mV. Glucose blocked the KATP channel and KCa
channel almost completely (Figure 3C).
|
Figure 3. A. Effect of glibenclamide on KATP and KCa
channels. Control. KATP channels were recorded at a membrane
potential of -50 mV. After application of glibenclamide (100 nmol/L).
Glibenclamide completely blocked KATP channels. B. Control.
The KCa channels were recorded at a membrane potential of 50 mV.
After application of glibenclamide (100 nmol/L). The activities of KCa
channels were not altered by application of glibenclamide (100 nmol/L). C.
Effects of 10 mmol/L glucose on ATP-sensitive K+ (KATP)
and Ca2+-dependent K+ (KCa) channel. Both KATP
(small conductance and long opening: arrow) and KCa (large
conductance and spiky opening) channels were seen before application of
glucose. The membrane potential was 50 mV. 10 mmol/L glucose blocked both KATP
and KCa channels almost completely. |
To clarify whether the glucose-induced decrease in KCa
channel activity was mediated by PKC, we treated the cells with staurosporine
(Figures 4 and 5). To increase KCa channel activity, 1 mmol/L of
A23187, a calcium ionophore, was used before the application of glucose.
Glucose significantly suppressed the KCa channel activity (Po:
0.221±0.224 to 0.014±0.023, P<0.05, n=7, Figure 4C). This effect was
significantly reversed by 1 nmol/L staurosporine (0.014±0.023 to 0.195±0.178,
P<0.05, n=7, Figure 4D).

|
Figure 4. Inhibitory effects of glucose on KCa channels. In this
experiment, the membrane potential was maintained at 40 mV. In control,
infrequent KCa channel activity was seen. The application of 1
mmol/L A23187 activated the KCa channels by increasing [Ca2+]i.
The activated KCa channels were blocked by 10 mmol/L glucose and
reactivated by 1 nmol/L staurosporine. To test whether a PKC activator could mimic the
effects of glucose on KCa channels, we tested the effect of PMA on
KCa channels (Figure 5). PMA significantly reduced KCa channel
activity (0.261±0.243 to 0.027±0.024, P<0.05, n=7). This inhibition was
also reversed by the application of 1 nmol/L staurosporine (0.027±0.024 to
0.147±0.122, P<0.05, n=7, Figure 5D). |
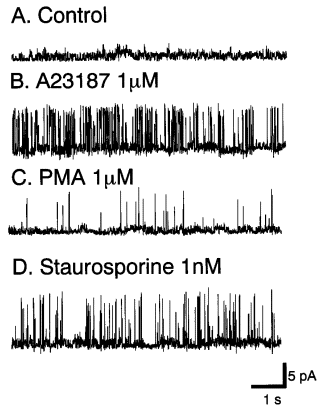
|
Figure 5. Inhibitory effects of PKC blockade on KCa channels. The
membrane potential was maintained at 40 mV. KCa channels were
activated by 1 mmol/L A23187 and 1 mmol/L. PMA significantly blocked these
channel activities. The KCa channels were reactivated by adding 1
nmol/L staurosporine to the same cell. |
DISCUSSION
The purpose of the present study was to clarify the
significance of PKC in the secretion of insulin induced by glucose.
Staurosporine, a PKC inhibitor, attenuated such secretion in insulinoma HIT
cells. In patch clamp studies, glucose blocked both the KCa and the
KATP channels. This blockade appeared to be mediated by PKC, since
staurosporine reversed the effect and PMA mimicked it. Staurosporine also
attenuated the increase in [Ca2+]i levels caused by
glucose. Thus, PKC contributed to the glucose-induced secretion of insulin at
least partly by blocking the KCa channels.
PKC is a calcium- and phospholipid-dependent enzyme
that is activated by the endogenous second messenger, diacylglycerol [22]. A
variety of approaches have been used to investigate the role of the activation
of PKC in stimulated secretion. The activation of PKC by such phorbol esters as
12-O-tetradecanoylphorbol-13-acetate (TPA) stimulates the secretion of insulin
in the absence or presence of basal levels of glucose (2.8 mmol/L) in rat islet
cells [23, 24] and insulinoma cells [16]. In our present study, staurosporine
partially suppressed the secretion of insulin by glucose, thus suggesting the
involvement of PKC.
Although PKC has been implicated in secretory
responses to glucose, its involvement in the regulation of nutrient-induced
insulin secretion is controversial. In isolated rat islet of Langerhans, which
had been pretreated with PMA for 20-24 h to deplete cells of PKC activity, Hii et
al. [25] concluded that PKC activation was not essential to the
glucose-induced secretion of insulin. However, in HIT cells, Hughes et al.
[26] found that PKC depletion blocked the acetylcholine-induced insulin release
as well as decreased the levels of insulin released in response to glucose. In
our studies, the glucose-induced insulin secretion was assayed not under the
PKC depleted condition but under the acute inhibition of PKC. In this
condition, staurosporine caused a slight decrease in glucose-induced insulin
secretion compared with that seen in cells stimulated by glucose alone. An
increased [Ca2+]i concentration is considered to be the
essential event that initiates glucose-induced insulin secretion, and the
activation of PKC reportedly increases [Ca2+]i levels.
Our study also showed that the inhibition of PKC by staurosporine slightly
decreased the [Ca2+]i levels. Therefore, the decrease in
glucose-induced insulin secretion was due to the diminution of [Ca2+]i
levels caused by the inhibition of PKC. In contrast with these data,
staurosporine had no effect on either glibenclamide-induced insulin secretion
or [Ca2+]i increase.
Resting HIT-T15 cells show two types of K+
channels, ATP-sensitive K+ channels (KATP) and Ca2+-
and voltage-activated K+ channels (KCa) as in normal
pancreatic beta-cells. KATP channels have an important role in
insulin secretion and KCa channels also play an important role in
beta-cell membrane repolarization after Ca2+ influx via the
voltage-gated Ca2+ channels. In our experiment, glibenclamide
blocked KATP channels selectively, leading to the increase of [Ca2+]i
and insulin secretion. It indicates that glibenclamide-induced insulin
secretion and [Ca2+]i increase come exclusively from the
blocking of KATP channels.
Phorbol ester, an activator of PKC, has previously
been shown to block K+ currents in several cell types [19]. The
ensuing activation of PKC due to an increase in the level of diacylglycerol
leads to the closure of KATP channels. In RINm5F cells, it was also
reported that PMA reduces KATP channel activity, leading to membrane
depolarization and an increase in [Ca2+]i levels [27].
HIT-T15 cells have the secretory properties of normal islets and respond to
nutrient secretagogues such as glucose. Eddkestone et al. demonstrated
that glucose depolarizes HIT cells by closing KATP channels [28].
Although HIT-T15 cells have been reported to decrease glucose-induced insulin
secretion as passage increases, it has both KATP and KCa
channels and they have properties very similar to normal beta cells. Thus, the
HIT cells appeared to be a valid model for the investigation. The present study
focused on KCa channels, so we did not study KATP
channels extensively. Our data showed that in the cell-attached patch
configurations, glucose and PMA inhibited the KCa channels that were
activated by increasing the [Ca2+]i levels by treatment
with A23187 (Figures 4 and 5). The PKC inhibitor staurosporine significantly
activated the KCa channels blocked either by glucose (Figure 4) or
PMA (Figure 5). These results indicate that the activation of PKC inhibits KCa
channels, probably via phosphorylation of the channel protein.
Received February 2nd, 2000 – Accepted May
5th, 2000
Key words Potassium Channels; Protein Kinase C; Staurosporine
Abbreviations KATP: ATP-sensitive K+; PKC:
protein kinase C: PMA: phorbol 12-myristate 13-acetate
Correspondence
Yutaka Nakaya
Department of Nutrition
School of Medicine
The University of Tokushima
3-18-15 Kuramoto-cho
Tokushima City
770-8503 Japan
Phone: +81-88.633.7090
Fax: +81-88.633.7113
E-mail address: nakaya@nutr.med.tokushima-u.ac.jp
References
1. Ashcroft
FM, Harrison DE, Ashcroft SJH. Glucose induces closure of single potassium
channels in isolated rat pancreatic beta-cells. Nature 1984; 312:446-8.
2. Cook DL,
Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B-cells.
Nature 1984; 311:271-3.
3. Rorsman P,
Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated
by intracellular ATP. Pflugers Arch 1985; 405:305-9.
4. Arkhammar
P, Nilsson T, Rorsman P, Berggren PO. Inhibition of ATP-regulated K+ channels
precedes depolarization-induced increase in cytoplasmic free Ca2+ concentration
in pancreatic beta-cells. J Biol Chem 1987; 262:5448-54.
5. Schmid-Antomarchi
HS, Weille JD, Fosset M, Lazdunski M. The receptor for antidiabetic
sulfonylureas controls the activity of the ATP-modulated K+ channel in
insulin-secreting cells. J Biol Chem 1987; 262:15840-4.
6. Henquin
JC, Charles S, Nenquin M, Mathot F, Tamagawa T. Diazoxide and D 600 inhibition
of insulin release. Diabetes 1982; 31:776-83.
7. Gembal M,
Gilon P, Henquin JC. Evidence that glucose can control insulin release
independently from its action on ATP-sensitive K+ channels in mouse B cells. J
Clin Invest 1992; 89:1288-95.
8. Gembal M,
Detimary P, Gilon P, Gao ZY, Henquin JC. Mechanisms by which glucose can
control insulin release independently from its action on adenosine
triphosphate-sensitive K+ channels in mouse B cells. J Clin Invest 1993;
91:871-80.
9. Aizawa T,
Sato Y, Ishihara F, Taguchi N, Komatsu M, Suzuki N, et al. ATP-sensitive K+
channel-independent glucose action in rat pancreatic b-cell. Am J Physiol 1994;
266:C622-7.
10. Berridge
MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular
signal transduction. Nature 1984; 312:315-21.
11. Streb H,
Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial
intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate.
Nature 1983; 306:67-9.
12. Nishizuka
Y. The role of protein kinase C in cell surface signal transduction and tumor
promotion. Nature 1984; 308:693-8.
13. Tanigawa
K, Kuzuya H, Imura H, Taniguchi H, Baba S, Takai Y, et al. Calcium-activated,
phospholipid-dependent protein kinase in rat pancreas islets of langerhans.
FEBS Lett 1982; 138:183-6.
14. Thams P,
Capito K, Hedeskov CJ. Endogenous substrate proteins for
Ca2+-calmodulin-dependent, Ca2+-phospholipid-dependent and cyclic AMP-dependent
protein kinases in mouse pancreatic islets. Biochem J 1984; 221:247-53.
15. Lord JM,
Ashcroft SJH. Identification and characterization of
Ca2+-phospholipid-dependent protein kinase in rat islets and hamster
beta-cells. Biochem J 1984; 219:547-51.
16. Hutton
JC, Peshavaria M, Brocklehurst KW. Phorbol ester stimulation of insulin release
and secretory-granule protein phosphorylation in a transplantable rat
insulinoma. Biochem J 1984; 224:483-90.
17. Deeney
JT, Cunningham BA, Chheda S, Bokvist K, Juntti-Berggren L, Lam K, et al.
Reversible Ca2+-dependent translocation of protein kinase C and glucose-induced
insulin release. J Biol Chem. 1996; 271:18154-60.
18. Nishizuka
Y. Studies and perspectives of protein kinase C. Science 1986; 233:305-12.
19. Higashida
H, Brown DA. Two polyphoshatidylinositide metabolites control two K+ currents
in a neuronal cell. Nature 1986; 323:333-5.
20. Findlay
I, Dunne MJ, Petersen OH. ATP-sensitive inward rectifier and voltage- and
calcium-activated K+ channels in cultured pancreatic islet cells. J Membr Biol
1985; 88:165-72.
21. Hamill
OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques
for high-resolution current recording from cells and cell-free membrane
patches. Pflugers Arch 1981; 391:85-100.
22. Nishizuka
Y. The family of protein kinase C for signal transduction. J Am Med Assoc 1989;
262:1826-33.
23. Malaisse WJ, Sener A, Herchuelz A,
Carpinelli AR, Poloczek P, Winand J, et al. Insulinotropic effect of the tumor promoter
12-O-tetradecanoylphorbol-13-acetate in rat pancreatic islets. Cancer Res 1980;
40:3827-31.
24. Malaisse
WJ, Lebrun P, Herchuelz A, Sener A, Malaisselagae F. Synergistic effect of a
tumor-promoting phorbol ester and a hypoglycemic sulfonylurea upon insulin
release. Endocrinology 1983; 113:1870-7.
25. Hii CST,
Jones PM, Persaud SJ, Howell SL. A re-assessment of the role of protein kinase
C in glucose-stimulated insulin secretion. Biochem J 1987; 246:489-93.
26. Hughes
SJ, Chalk JG, Ashcroft SJH. The role of cytosolic free Ca2+ and protein kinase
C in acetylcholine-induced insulin release in the clonal beta-cell line,
HIT-T15. Biochem J 1990; 267:227-32.
27. Wollheim
CB, Dunne MJ, Peter-Riesch B, Bruzzone R, Pozzan T, Petersen OH. Activators of
protein kinase C depolarize insulin-secreting cells by closing K+ channels.
EMBO J 1988; 7:2443-9.
28. Eddlestone
GT, Ribalet B, Ciani S. Comparative study of K channel behavior in beta cell
lines with different secretory responses to glucose. J Membr Biol 1989;
109:123-34.