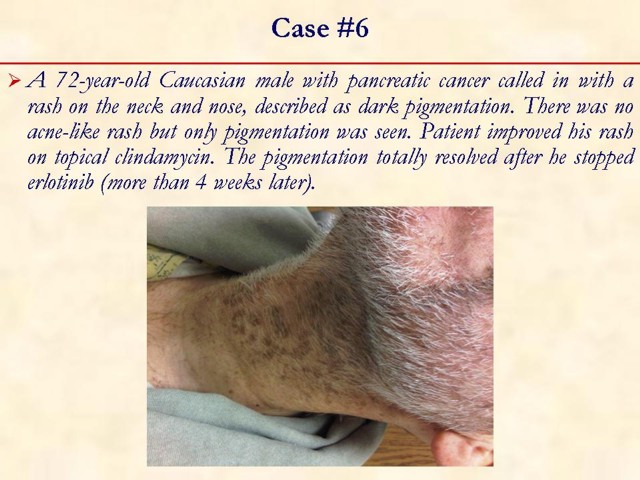
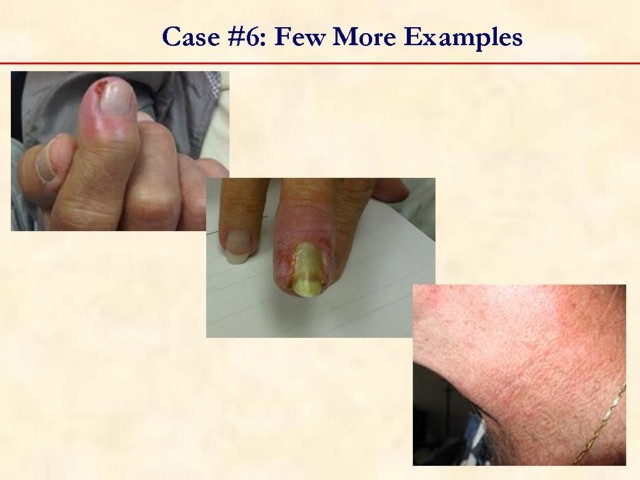
MULTIMEDIA ARTICLE - Slide Show
JOP. J Pancreas (Online) 2010 Mar 5; 11(2):176-182.
Management of Skin Toxicities of Anti-EGFR Agents in Patients with Pancreatic Cancer and Other GI Tumors by Using Electronic Communication: Effective and Convenient
Muhammad Wasif Saif, Kristin Kaley, Lynne Lamb, Jennifer Pecerillo, Susan Hotchkiss, Lisa Steven, Marianne Brennan, Robin Penney, Carolyn Gillespie, Walid Shaib
Yale University School of Medicine. New Haven, CT, USA
Summary
Erlotinib has been FDA approved to be used in combination with gemcitabine as the first line treatment in advanced pancreatic cancer patients. Skin rash has been documented as one of the commonest adverse reactions in patients receiving erlotinib and other EGFR inhibitors. Draw back to this reaction leads to: 1) drug discontinuation or dose reduction; 2) impairs quality of life; and 3) Puts patients at risk of superinfection. Monitoring patients closely and initiating immediate skin care is recommended. However, patients forget how the rash started and when. No standard treatments exist secondary to the diversity of symptoms, variability and intermittent occurrence in relation to the cancer therapy. In addition, there is slow improvement with medical treatment. Also, patients need to make extra visits to doctor’s office for skin management when in needed in addition to chemotherapy appointments. Late presentation for medical attention leading to complications, such as sepsis. We here experience a novel way of assessing and managing the skin rash using the electronic media. We suggest that electronic communication is of crucial importance to detect early, diagnose and treat anti-EGFR related skin rash in order to continue the benefit of anti-EGFR.
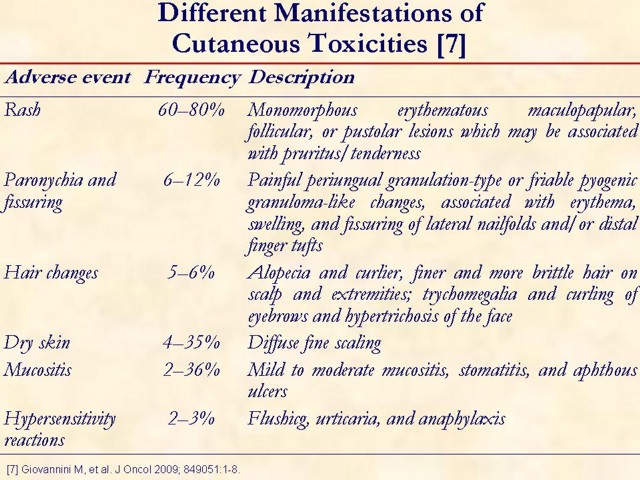
Secondary adverse reactions seen with anti-EGFR therapy include xerosis, pruritus, paronychia, hair abnormality, and mucositis [2].
A phase III randomized controlled trial by National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) has shown a statistically significant survival benefit of gemcitabine plus erlotinib compared with gemcitabine alone. The combined treatment arm demonstrated an 18% reduction in the risk of death or an overall 22% improvement in survival than the gemcitabine alone arm, and it was statistically superior in 1-year survival (23.8% vs. 19.4%; P=0.028) and in median survival (6.4 vs. 6.0 months) [3].
The rash develops as early as three days after commencement of erlotinib therapy, with median onset of eight days [4].
Erlotinib, a small-molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor has been approved by FDA for patients with pancreatic cancer and non-small cell lung cancer [1].
Skin toxicity may lead to drug discontinuation or dose reduction, impair patients’ activities and exposes the skin to bacterial infections. Preservation of quality of life in these patients is crucial [1].
Toxicity is seen in at least 79% patients treated with erlotinib [5].
Grade 3-4 rash was documented in 9% of erlotinib treated patients, requiring dose reduction in 6% and discontinuation in 1% of patients [6].

Key point: skin rash can be managed with appropriate intervention.
Skin rash occurred in 71% (grade 1-2: 66%; grade 3: 3%; grade 4: 2%); median time of onset was 10 days (range: 1-44 days) [9].

The dermatologic reactions from anti-EGFR agents, which include antibodies against the extracellular ligand-binding domain of the receptor and small molecules that inhibit activation of the EGFR-tyrosine kinase are commonly found in sites where EGFR is expressed, such as the basal epidermal keratinocytes of epidermis, sebaceous glands, and hair follicles. Histopathological findings of the skin lesion reveal folliculitis and perifolliculitis with a diffuse neutrophilic infiltrate in the dermis. It has been speculated that cutaneous toxicity from anti-EGFR therapy may be a result of an inflammatory response secondary to EGFR inhibition and/or decreased keratinocyte proliferation/maturation. Markers in the epidermal growth factor receptor pathway and skin toxicity during erlotinib treatment [10].

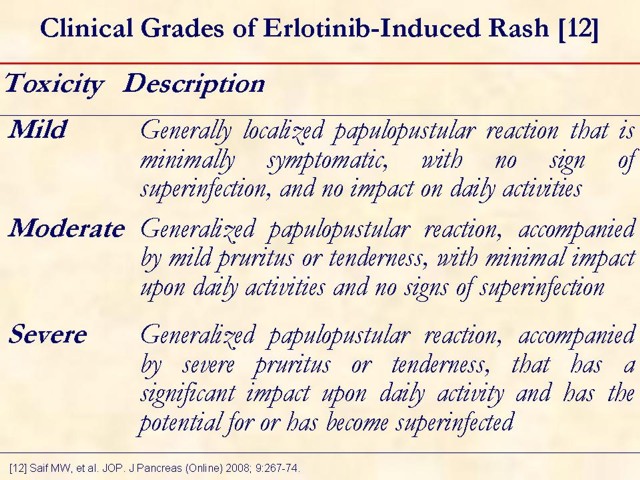
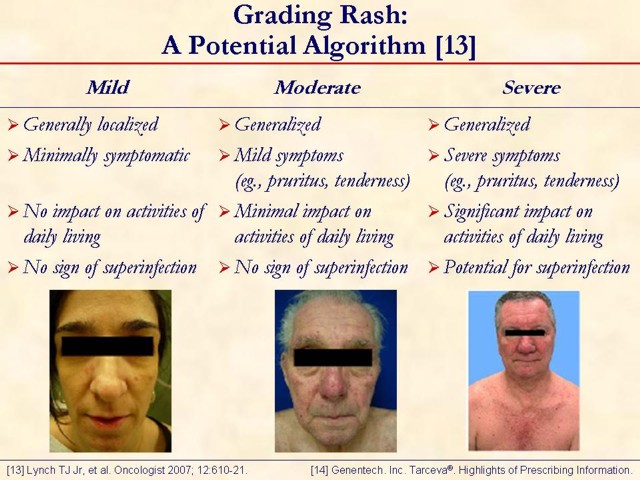
This slide shows mild, moderate, and severe rash in patients treated with erlotinib. This grading system should not be construed as per Genentech, Inc. (South San Francisco, CA, USA) or OSI Pharmaceuticals, Inc. (Long Island, NY, USA) recommendations [13, 14]. It was developed by medical advisors at the "Skin Toxicity Forum" held in Chicago, Illinois, during October 2006 [13]. These medical advisors were paid by Genentech, Inc., OSI Pharmaceuticals, Inc., and F. Hoffmann-La Roche AG (Basel, Switzerland), to participate in the forum. Other medical experts may have a different approach to managing rash.
Rash typically appears on the face and/or upper body in varying degrees and tolerability. For some, severe rash was tolerable; for others, mild rash was intolerable. The rash associated with erlotinib treatment is not acne, though its appearance is similar to acne. Rash varies in presentation and degree. An interactive discussion regarding grading is encouraged to demonstrate the subjective nature of EGFR rash grading currently used in clinical practice.

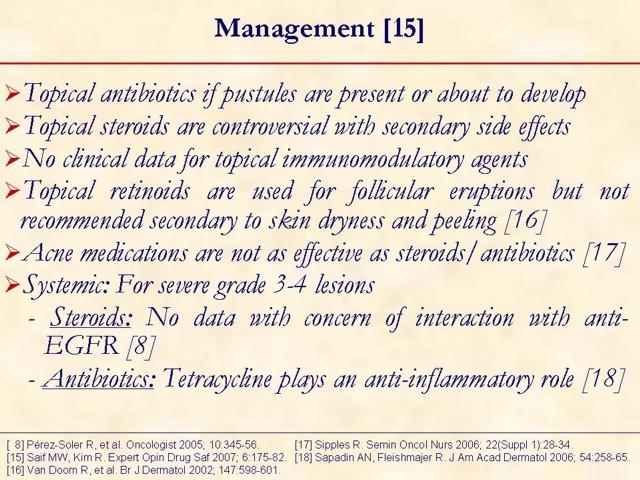
Key points: i) skin rash can be managed with appropriate intervention; ii) erlotinib should be taken at least one hour before or two hours after the ingestion of food.
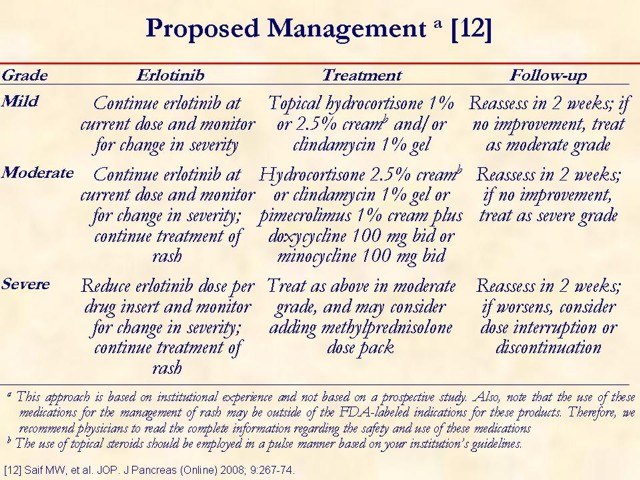
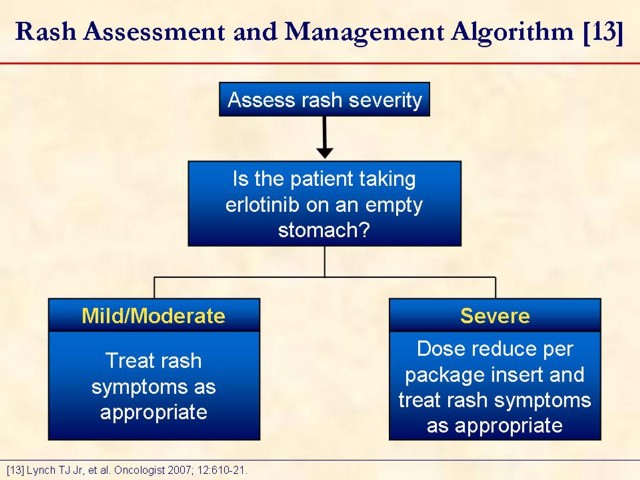
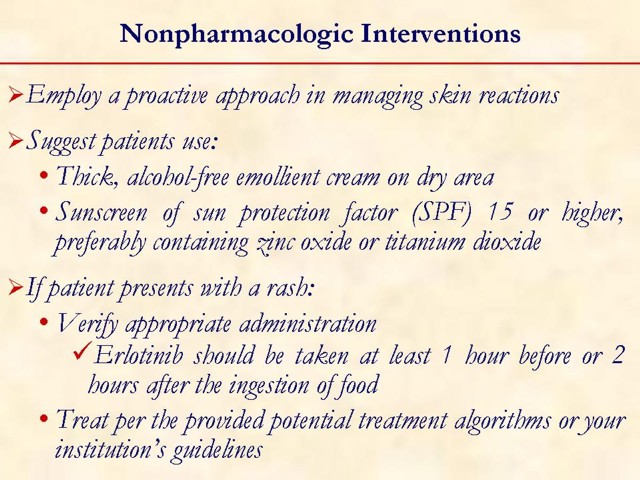
Key point: erlotinib should be taken at least one hour before or two hours after the ingestion of food. This slide is designed to open a dialogue among attendees on how they manage rash in their practice. Measures: they take upfront, such as patient education initiatives and prophylactic measures, should be discussed. Management options, once a patient develops a rash while on erlotinib, should be discussed as well.
· Do they dose reduce erlotinib? Why?
· Do they discontinue erlotinib?
· Do they modify the erlotinib regimen?
· Do they maintain erlotinib at the current dose and treat the rash, and if so, how?

Nimotuzumab, a humanized murine mAb created in Cuba, has demonstrated antitumor activity similar to that of other anti-EGFR mAbs and shows promise as a single agent and as an adjunct to radiation in Phase I and II clinical trials. Surprisingly, the typical severe dermatological toxicities thus far associated with anti-EGFR therapy have not been described with nimotuzumab [21].
Cetuximab, erlotinib, and gefitinib have been approved for patients with colorectal and non-small cell lung cancer refractory or intolerant to chemotherapy. The most commonly encountered adverse effect was a mild skin toxicity characterized by a sterile follicular and pustular rash that may be treated empirically and usually does not require treatment modification. Although the precise mechanism for development of rash is not well defined, it is related to inhibition of EGFR-signaling pathways in the skin, and may serve as visible markers of anti-tumor activity and therapeutic efficacy [2].

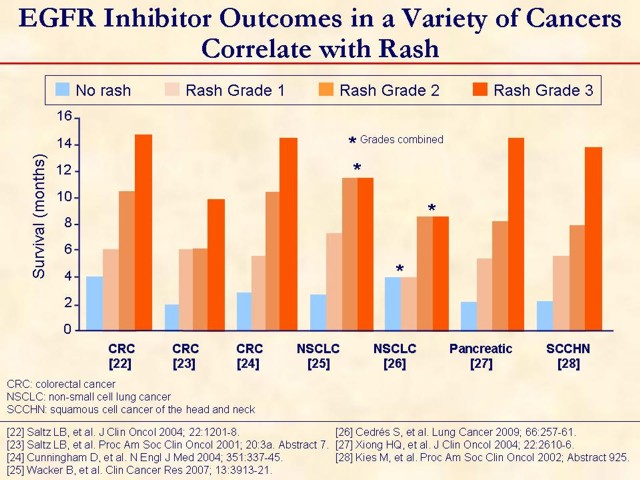
All of the above are retrospective. All are with cetuximab except Wacker et al. [25] and Cedrés et al. [26] are with erlotinib. There are similar analyses that did not find the correlation. Should be interpreted with caution due to potential bias that exists because patients with a naturally longer life expectancy would be on the EGFR inhibitor longer and therefore be more likely to develop the rash.


New communication technologies must never replace the crucial interpersonal contacts that are the very basis of the patient-physician relationship. Rather, electronic mail and other forms of Internet communication should be used to enhance such contacts.


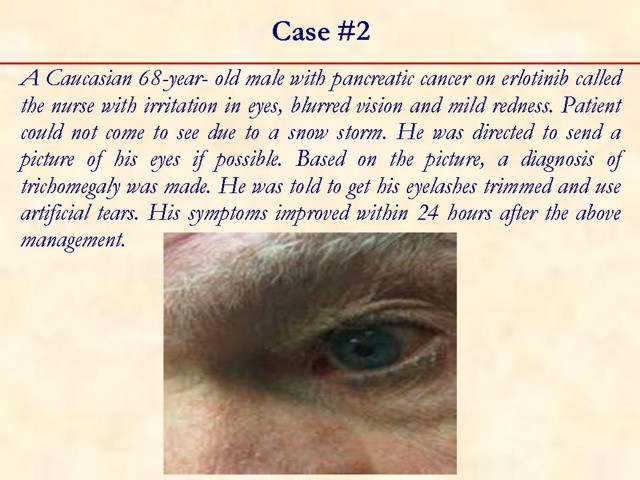
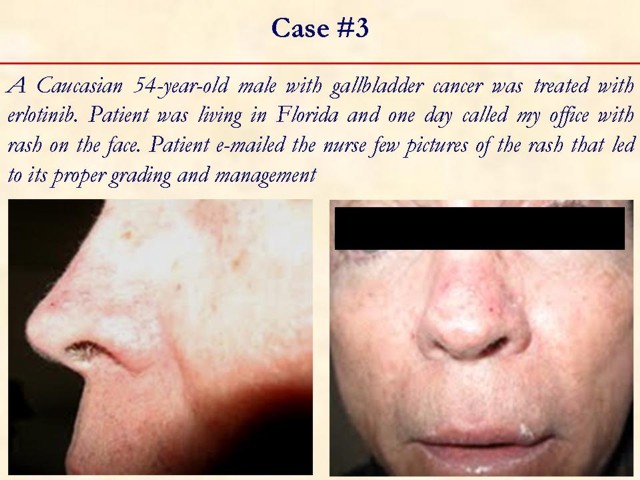

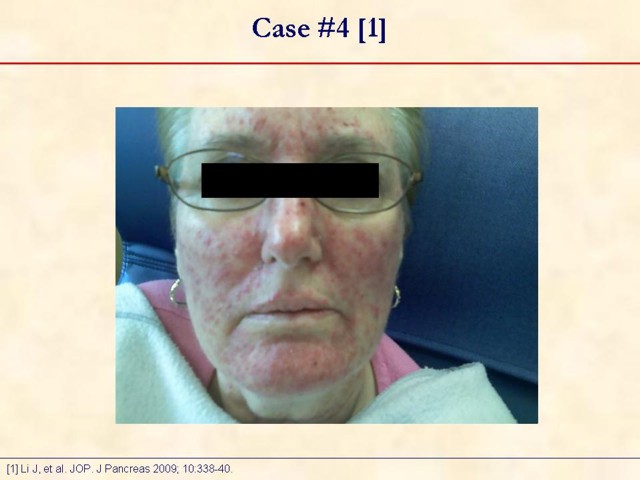
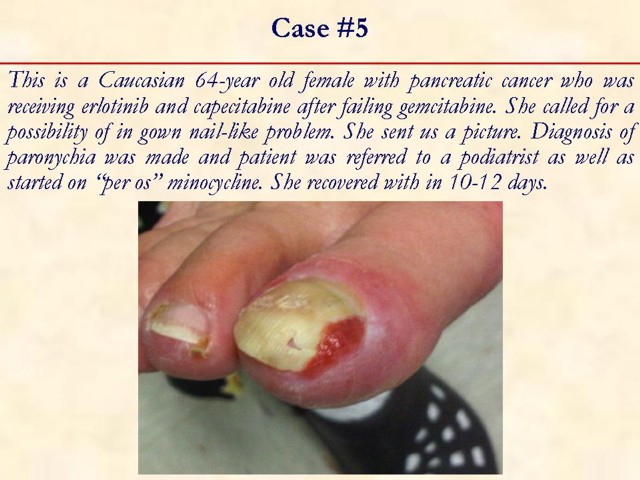


Received January 14th, 2010 - Accepted January 24th, 2010
Key words cetuximab; Drug Therapy; Epidermal Growth Factor; erlotinib; Pancreatic Neoplasms; panitumumab; Protein Kinase Inhibitors; Receptor, Epidermal Growth Factor
Abbreviations EGFR: Epidermal Growth Factor Receptor, NCI-CTCAE: National Cancer Institute: Common Terminology Criteria for Adverse Events; FDA: Food and Drug Administration
Conflict of interest The authors have no potential conflicts of interest
Correspondence
Muhammad
Wasif Saif
Section of Medical Oncology
Yale University School of Medicine
333 Cedar Street; FMP: 116
New Haven, CT 06520
USA
Phone: +1-203.737.1568
Fax:+1-203.785.3788
E-mail: wasif.saif@yale.edu
References
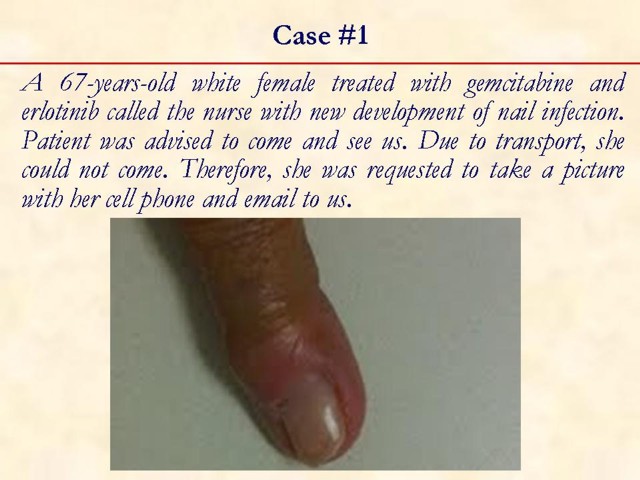
1. Li J, Peccerillo J, Kaley K, Saif MW. Staphylococcus aureus bacteremia related with erlotinib skin toxicity in a patient with pancreatic cancer. JOP. J Pancreas (Online) 2009; 10:338-40. [PMID 19454833] (FULL TEXT: http://www.joplink.net/prev/200905/22.html)
2. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006; 55:657-70. [PMID 17010747] (FULL TEXT: http://www.eblue.org/article/PIIS0190962205032408/fulltext)
3. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25:1960-6. [PMID 17452677] (FULL TEXT: http://jco.ascopubs.org/cgi/content/full/25/15/1960)
4. Boeck S, Hausmann A, Reibke R, Schulz C, Heinemann V. Severe lung and skin toxicity during treatment with gemcitabine and erlotinib for metastatic pancreatic cancer. Anticancer Drugs 2007; 18:1109-11. [PMID 17704662] (FULL TEXT: http://journals.lww.com/anti-cancerdrugs/pages/articleviewer.aspx?year=2007&issue=10000&article=00015&type=abstract)
5. Saif MW. Pancreatic cancer: highlights from the 42nd annual meeting of the American Society of Clinical Oncology, 2006. JOP. J Pancreas (Online) 2006; 7:337-48. [PMID 16832131] (FULL TEXT: http://www.joplink.net/prev/200607/02.html)
6. Gutzmer R, Werfel T, Kapp A, Elsner J. Cutaneous side effects of EGF-receptor inhibition and their management. Hautarzt 2006; 57:509-13. [PMID 16205868] (FULL TEXT: http://www.springerlink.com/content/y4706p1871226870/fulltext.html)
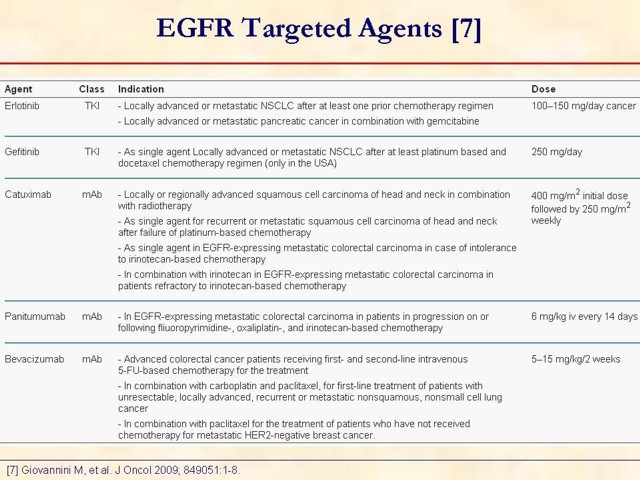
7. Giovannini M, Gregorc V, Belli C, Roca E, Lazzari C, Viganò MG, et al. Clinical significance of skin toxicity due to EGFR-targeted therapies. J Oncol 2009; 849051:1-8. [PMID 19584908] (FULL TEXT: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2699661/?tool=pubmed)
8. Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist 2005; 10:345-56. [PMID 15851793] (FULL TEXT: http://theoncologist.alphamedpress.org/cgi/content/full/10/5/345)
9. Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 2004; 22:77-85. [PMID 14701768] (FULL TEXT: http://jco.ascopubs.org/cgi/content/full/22/1/77)
10. Tan AR, Steinberg SM, Parr AL, Nguyen D, Yang SX. Markers in the epidermal growth factor receptor pathway and skin toxicity during erlotinib treatment. Ann Oncol 2008; 19:185-90. [PMID 17878175] (FULL TEXT: http://annonc.oxfordjournals.org/cgi/content/full/19/1/185)
11. National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). CTEP: Cancer Therapy Evaluation Program. Publish date August 9, 2006 Accessed January 14, 2008. (FULL TEXT: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf)
12. Saif MW, Merikas I, Tsimboukis S, Syrigos K. Erlotinib-induced skin rash. Pathogenesis, clinical significance and management in pancreatic cancer patients. JOP. J Pancreas (Online) 2008; 9:267-74. [PMID 18469438] (FULL TEXT: http://www.joplink.net/prev/200805/01.html)
13. Lynch TJ Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 2007; 12:610-21. [PMID 17522250] (FULL TEXT: http://theoncologist.alphamedpress.org/cgi/content/full/12/5/610)
14. Genentech. Inc. Tarceva®. Highlights of Prescribing Information. Accessed February 19, 2007. (FULL TEXT: http://www.gene.com/gene/products/information/pdf/tarceva-prescribing.pdf)
15. Saif MW, Kim R. Incidence and management of cutaneous toxicities associated with cetuximab. Expert Opin Drug Saf 2007; 6:175-82. [PMID 17367263] (FULL TEXT: http://informahealthcare.com/doi/full/10.1517/14740338.6.2.175)
16. Van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G. Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol 2002; 147:598-601. [PMID 12207609] (FULL TEXT: http://www3.interscience.wiley.com/cgi-bin/fulltext/118938563/HTMLSTART)
17. Sipples R. Common side effects of anti-EGFR therapy: acneform rash. Semin Oncol Nurs 2006; 22(Suppl 1):28-34. [PMID 16616284] (FULL TEXT: http://www.seminarsoncologynursing.com/article/PIIS0749208106000362/fulltext)
18. Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol 2006; 54:258-65. [PMID 16443056] (FULL TEXT: http://www.eblue.org/article/PIIS0190962205032317/fulltext)
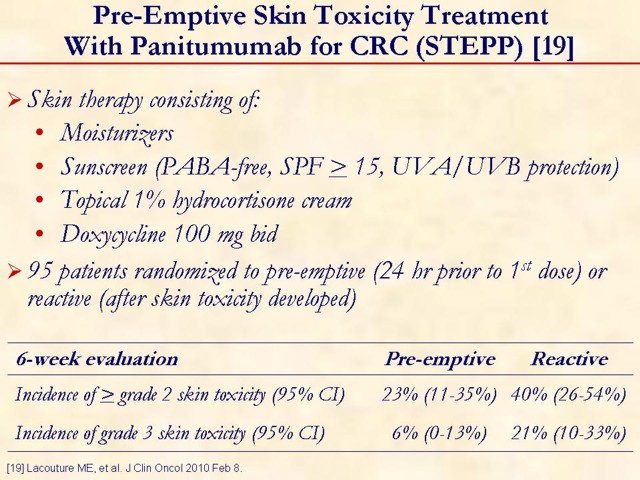
19. Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol 2010 Feb 8. [PMID 20142600] (FULL TEXT: http://jco.ascopubs.org/cgi/reprint/JCO.2008.21.7828v1)
20. Saif MW, Cohenuram M. Role of panitumumab in the management of metastatic colorectal cancer. Clin Colorectal Cancer 2006; 6:118-24. [PMID 16945167] (FULL TEXT: http://cigjournals.metapress.com/content/b40383qphu01244t/fulltext.pdf)
21. Boland WK, Bebb G. Nimotuzumab: a novel anti-EGFR monoclonal antibody that retains anti-EGFR activity while minimizing skin toxicity. Expert Opin Biol Ther 2009; 9:1199-206. [PMID 19624281] (FULL TEXT: http://informahealthcare.com/doi/full/10.1517/14712590903110709)
22. Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004; 22:1201-8. [PMID 14993230] (FULL TEXT: http://jco.ascopubs.org/cgi/content/full/22/7/1201)
23. Saltz LB, Rubin M, Hochster H, et al. Cetuximab (IMC-225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFR). Proc Am Soc Clin Oncol 2001; 20:3a. Abstract 7.
24. Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004; 351:337-45. [PMID 15269313] (FULL TEXT: http://content.nejm.org/cgi/content/full/351/4/337)
25. Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007;13:3913-21. [PMID 17606725] (FULL TEXT: http://clincancerres.aacrjournals.org/content/13/13/3913.full)
26. Cedrés S, Prat A, Martínez P, Pallisa E, Sala G, Andreu J, et al. Clinical surrogate markers of survival in advanced non-small cell lung cancer (NSCLC) patients treated with second-third line erlotinib. Lung Cancer 2009; 66:257-61. [PMID 19231023] (FULL TEXT: http://www.lungcancerjournal.info/article/PIIS0169500209000543/fulltext)
27. Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II trial. J Clin Oncol 2004; 22:2610-6. [PMID 15226328] (FULL TEXT: http://jco.ascopubs.org/cgi/content/full/22/13/2610)
28. Kies M, Arquette MA, Nabell L, et al. Final report of the efficacy and safety of the anti-epidermal growth factor antibody Cetuximab (IMC-C225), in combination with cisplatin in patients with recurrent squamous cell carcinoma of the head and neck (SCCHN) refractory to cisplatin containing chemotherapy. Proc Am Soc Clin Oncol 2002; Abstract 925.
29. Kane B, Sands DZ. White paper: guidelines for the clinical use of electronic mail with patients. J Am Med Inform Assoc 1998; 5:104-11. [PMID 9452989] (FULL TEXT: http://jamia.bmj.com/content/5/1/104.full)