CASE REPORT
JOP. J Pancreas (Online) 2012 Mar 10; 13(2):222-225.
Rare Cause of Delayed Upper Gastrointestinal Bleeding After Pancreaticoduodenectomy
Aneel Damle, Asa Clemenzi-Allen, Nicolas Jabbour, Shimul A Shah
Solid Organ Transplantation, Department of Surgery, University of Massachusetts Medical School. Worcester, MA, USA
ABSTRACT
Context Luminal bleeding after pancreaticoduodenectomy can be present in various degrees of acuity in up to 30% of patients. Case report In this report, we describe a rare and uncommon cause of gastrointestinal bleeding after pancreaticoduodenectomy and review of the literature. Conclusions Multiple biliary procedures with common complications increase the difficulty making the correct diagnosis and therefore all possible etiologies of a complication must be evaluated.
INTRODUCTION
In today’s multidisciplinary approach to medicine, complications can arise from multiple interventions in a patient’s care. It is critical to understand the possible complications of each intervention a patient undergoes to determine the correct diagnosis and direct appropriate treatment.
Upper gastrointestinal bleeds are commonly encountered in the clinical setting. The following presentation is a patient with life threatening gastrointestinal bleeding after pancreaticoduodenectomy. It serves as a reminder to evaluate all possible complications of previous interventions as well as the role of angiography in diagnosis and treatment of hemobilia.
CASE REPORT
The patient is a 68-year-old man who presented with jaundice, weight loss, abdominal pain, and early satiety. He had a recent history of gallstone pancreatitis, post ERCP with sphincterotomy and common bile duct stent placement and cholecystectomy. He had been discharged home in good condition, but returned due to a severe gastric outlet obstruction. He underwent repeat ERCP, but the procedure was aborted secondary to a duodenal stenosis. His biliary system was then drained with a percutaneous biliary drainage with a 8-Fr internal-external biliary drain (Flexima®, Boston Scientific, Natick, MA, USA). Figure 1a demonstrates a lack of contrast filling the duodenum by percutaneous transhepatic cholangiography (PTC), and Figure 1b demonstrates the placement of the drain. An endosonography was then performed which confirmed a mass in the head of the pancreas. This was found by fine needle aspiration to be pancreatic adenocarcinoma with tumor invading into the duodenum. A staging helical abdominal CT with pancreatic protocol was performed, and one week later the patient underwent a classic pancreaticoduodenectomy with distal gastrectomy. At the end of surgery the percutaneous biliary drain was removed. The patient tolerated the procedure well. On post-operative day number three, the patient became acutely hypotensive and had a melanotic stool with a drop in hemoglobin from 9.0 g/dL pre-operatively to 6.4 g/dL (reference range: 12.0-16.0 g/dL) requiring transfusion of 3 units of packed red blood cells. Endoscopy on two consecutive days identified the gastric anastomosis but found only clotted blood in the stomach with no source of bleeding. The patient’s hematocrit later stabilized, and he was discharged home on postoperative day seven.
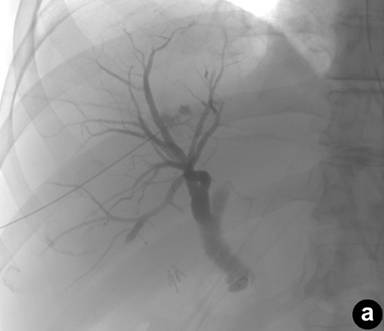
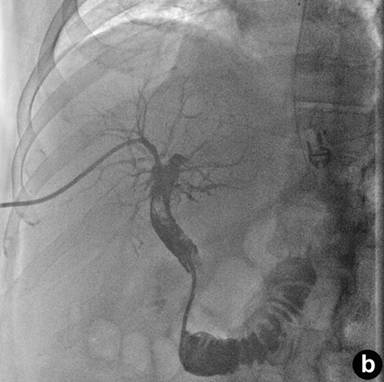
|
Figure 1. a. Percutaneous transhepatic cholangiography with filling defect of the duodenum. b. Percutaneous biliary drainage placement and filling of the duodenum. |
The patient returned three weeks later with near-syncopal symptoms, melena, and hematochezia. As the patient was exhibiting signs of intra-luminal bleeding, his first line diagnostic method was endoscopy which had to be repeated the following day due to clotted blood in the stomach. An additional 9 units of packed red blood cells were transfused. A tagged red-blood cell scan demonstrated bleeding localized to the proximal small bowel, and angiography demonstrated a right intra-hepatic artery pseudoaneurysm (Figure 2ab). The appearance of this pseudoaneurysm was small and intrahepatic and there was no extravasation of contrast or communication with the biliary tree. However, this was the likely source of bleeding and therefore we proceed to embolize it expeditiously. The patient had two straight 2 mm microcoils and two vortex microcoils of 3 ad 3.3 mm placed for selective embolization. After the procedure, the patient’s hematocrit stabilized, and he had no further episodes of hypotension or gastrointestinal bleeding. He is currently doing well without any sequelae nine months after surgery.
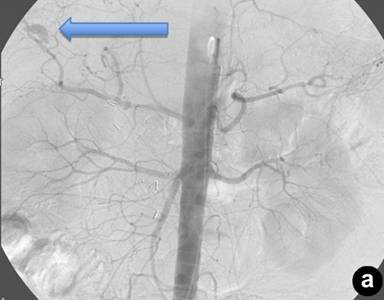
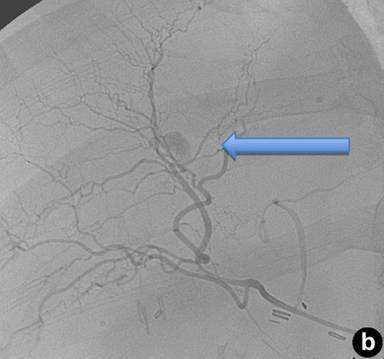
|
Figure 2. Right intra-hepatic artery pseudoaneurysm. Note the proximity of the percutaneous transhepatic cholangiography catheter (a.) to the location of the pseudoaneurysm (b.). |
DISCUSSION
Gastrointestinal bleeding is a widely known complication after pancreaticoduodenectomy with a reported incidence of 2-18% [1]. Etiologies of gastrointestinal bleeding range from technical failure at the anastomotic site to marginal ulcer formation to visceral artery bleeding (Table 1) [2]. As there have been a wide range of published bleeding rates after pancreaticoduodenectomy, the International Study Group of Pancreatic Surgery (ISGPS) proposed a classification system of post-pancreatic hemorrhage based on time of onset, location of bleeding, and severity of hemorrhage. Onset is defined as early (less than 24 hours) versus delayed (more than 24 hours) to more accurately classify causes of bleeding [11].
|
Table 1. Causes of gastrointestinal bleed after pancreaticoduodenectomy. |
||||
|
Study |
Subjects |
Total bleeds |
Gastrointestinal bleeds |
Causes (% of all patients) |
|
Limongelli et al. [3] a |
4,333 |
169 (3.9%) |
62 (1.4%) |
Pseudoaneurysm: 53 (1.2%) |
|
Rumstadt et al. [4] |
559 |
42 (7.5%) |
22 (3.9%) |
Pseudoaneurysm: 1 (0.2%) |
|
Choi et al. [5] |
500 |
22 (4.4%) |
20 (4.0%) |
Anastomotic sites: 3
(0.6%) |
|
Rajarathinam et al. [6] a |
458 |
14 (3.1%) |
6 (1.3%) |
Arterial bleeding in
pancreas: 5 (1.1%) |
|
Yekebas et al. [7] |
1,524 |
87 (5.7%) |
36 (2.4%) |
Vascular erosions: 13
(0.9%) |
|
de Castro et al. [8] a |
1,010 |
23 (2.3%) |
14 (1.4%) |
Sepsis/leak: 17 (1.7%) |
|
Fujii et al. [9] |
357 |
13 (3.6%) |
Not reported |
Pseudoaneurysm: 7 (2.0%) |
|
Treckmann et al. [10] a |
189 |
11 (5.8%) |
6 (3.2%) |
Arterial: 9 (4.8%) |
|
a Measured only delayed hemorrhage |
||||
The most common cause of early post-pancreatic hemorrhage is technical failure at the anastomotic site. Causes of delayed post-pancreatic hemorrhage are pancreatic leak leading to vessel disruption, intra-abdominal infection with peripancreatic involvement, and vascular injury during resection [11]. Location of bleeding is defined as intra-luminal versus extra-luminal. Finally, severity of hemorrhage is evaluated as mild versus severe. Mild hemorrhage consists of a drop in hemoglobin less than 3 g/dL without physiologic impairment and successful conservative management consisting of i.v. fluids and transfusion of less than 3 units of blood. Severe hemorrhage is defined as a hemorrhage equal to, or greater than, 3 g/dL, causing clinical impairment, and requiring more than 3 units of blood and/or invasive operative or angiographic therapy. The ISGPS has combined the three factors above to classify hemorrhage as grade A, B, or C [11]. In our patient, the first episode of bleeding was a grade A hemorrhage (least severe), and the second episode was classified as a grade C (most severe) hemorrhage.
A meta-analysis evaluating delayed post-operative hemorrhage after pancreaticoduodenectomy demonstrated pseudoaneurysm to be involved in 31% of cases [4]. The mechanism of pseudoaneurysm is thought to be related to pancreatic leak causing irritation to the adventitia of the arterial wall. A common associated finding with bleeding from a visceral artery pseudoaneurysm is the sentinel bleed days to weeks before a large gastrointestinal hemorrhage [10]. A single center retrospective review of 35 cases of delayed post-pancreatic hemorrhage secondary to pseudoaneurysm demonstrated a sentinel bleed in 45% of patients [12].
Hemobilia is a known complication of percutaneous biliary instrumentation. Percutaneous liver interventions are the leading cause of iatrogenic hemobilia [13]. The quality improvement guidelines for PTC and biliary drainage published by the Journal of Vascular and Interventional Radiology recognize hemorrhage as a complication of these procedures and cite a 2.5% complication rate and suggest an acceptable hemorrhage rate of 5% [14]. A comparison of left sided versus right sided PTDB was performed on a series of 346 patients and showed an increased rate of hemobilia in left sided biliary drainage procedures; however, it was not shown to be a statistically significant increase over the right side [15].
Percutaneous biliary drainage can cause immediate bleeding by direct vascular injury or a delayed bleed secondary to pseudoaneurysm formation with communication to the biliary system [16]. A case of intrahepatic pseudoaneurysm causing life-threatening upper gastrointestinal bleed immediately after removal of a biliary drainage catheter has been reported in the literature [17]. The suspected cause was the 18-Fr drainage catheter injuring the wall of the adjacent left hepatic artery, which tamponaded the site until the time of drain removal. In our case, there was a sentinel bleed which then subsided leading us to believe this was an enteric staple line bleed from the gastric anastomosis. When the patient represented with bleeding three weeks after the surgery, it was unclear about the pathogenesis given the timing. When repeated endoscopies are not conclusive, a four-phase CT scan or angiogram should be obtained.
When patients have percutaneous biliary drainage prior to pancreaticoduodenectomy, what is the optimal timing for removal of the drain? Many would consider 4-6 weeks the optimal timing to provide ample drainage for the biliary anastomosis. In the setting of cancer with the biliary tube in the duodenum, it was our approach to remove it at the time of surgery since there may have been potential for cancer seeding. Since this was a delayed bleed, the cause of bleeding would remain a diagnostic dilemma even after pulling the drain out six weeks later; conversely, the differential diagnosis of bleeding is significantly narrowed with the delayed removal since early causes of gastrointestinal bleeding have been ruled out.
SUMMARY
Gastrointestinal hemorrhage is a known complication of both pancreaticoduodenectomy and percutaneous biliary drainage. While bleeds within 24 hours are likely associated with anastomotic bleeding, causes for bleeding after the 24-hour time period are more variable [1]. Multiple procedures with common complications add difficulty to the diagnosis and therefore all possible etiologies of a complication must be evaluated.
Received October 4th, 2011 - Accepted January 2nd, 2012
Key words Biliary Tract Surgical Procedures; Hemobilia; Hemorrhage; Jaundice; Pancreatic Neoplasms; Pancreaticoduodenectomy
Abbreviations ISGPS: International Study Group of Pancreatic Surgery
Conflict of interest All authors have no disclosures or conflicts of interest to report
Correspondence
Shimul
A Shah
Solid Organ Transplantation
Department of Surgery
University of Massachusetts Medical School
55 Lake Avenue North, S6-432
Worcester, MA 01655
USA
Phone: +1-508.334.2023
Fax: +1-508.856.1102
E-mail: shimul.shah@umassmemorial.org
References
1. Roulin D, Cerantola Y, Demartines N, Schäfer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg 2011; 15:1055-62. (More details: [1]).
2. Schäfer M, Heinrich S, Pfammatter T, Clavien PA. Management of delayed major visceral arterial bleeding after pancreatic surgery. HPB 2011; 13:132-8. (More details: [2]).
3. Limongelli P, Khorsandi SE, Pai M, Jackson JE, Tait P, Tierris J, Habib NA, Williamson RCN, Jiao LR. Management of delayed postoperative hemorrhage after pancreaticoduodenectomy: a meta-analysis. Arch Surg 2008; 143(10):1001-7. (More details: [3]).
4. Rumstadt B, Schwab M, Korth P, Samman M, Trede M. Hemorrhage after pancreatoduodenectomy. Ann Surg 2008; 227(2):236-41. (More details: [4]).
5. Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg 2004; 199(2):186-91. (More details: [5]).
6. Rajarathinam G, Kannan DG, Vimalraj V, Amudhan A, Rajendran S, Jyotibasu D, et al. Post pancreaticoduodenectomy haemorrhage. HPB 2008; 10(5):363-70. (More details: [6]).
7. Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski DR, Koenig AM, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007; 246(2):269-80. (More details: [7]).
8. de Castro SM, Kuhlmann KF, Busch OR, van Delden OM, Laméris JS, van Gulik TM, et al. Delayed massive hemorrhage after pancreatic and biliary surgery embolization or surgery? Ann Surg 2005; 241(1):85-91. (More details: [8]).
9. Fujii Y, Shimada H, Endo I, Yoshida K, Matsuo K, Takeda K, et al. Management of massive arterial hemorrhage after pancreatobiliary surgery: does embolotherapy contribute to successful outcome? J Gastrointest Surg 2007; 11(4):32-438. (More details: [9]).
10. Treckmann J, Paul A, Sotiropoulos GC, Lang H, Özcelik, Saner AF, Broelsch CE. Sentinel bleeding after pancreaticoduodenectomy: a disregarded sign. J Gastrointest Surg 2008; 12(2):313-8. (More details: [10]).
11. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy haemorrhage (PPH) - an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery 2007; 142:20-25. (More details: [11]).
12. Ding X, Zhu J, Zhu M, Li C, Jian W, Jiang J, Wang Z, Hu S, Jiang X. Therapeutic management of hemorrhage from visceral artery pseudoaneurysms after pancreatic surgery. J Gastrointest Surg 2011 (Oline May 17, 2011) (More details: [12]).
13. Chin MW, Enns R. Hemobilia. Curr Gastroenterol Rep 2010; 12:121-9. (More details: [13]).
14. Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, et al. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. Society of cardiovascular and interventional radiology. J Vasc Interv Radiol 1997; 8(4):677-81. (More details: [14]).
15. Rivera-Sanfeliz GM, Assar OSA, LaBerge JM, Wilson MW, Gordon RL, Ring EJ, Kerlan RK. Incidence of Important Hemobilia following transhepatic biliary drainage: left-sided versus right-sided approaches. Cardiovasc Intervent Radiol 2004; 27:137-9. (More details: [15]).
16. Fidelman N, Bloom AI, Kerlan RK, LaBerge JM, Wilson MW, Ring EJ, Gordon RL. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology 2006; 247(3):880-6. (More details: [16]).
17. Taneja M, Lo R, Sebastian MG, Chow PKH. Intra-hepatic arterial PSA causing life-threatening upper GI bleed after removal of biliary drainage catheter. Biomed Imaging Interv J 2009; 5(3):e20. (More details: [17]).